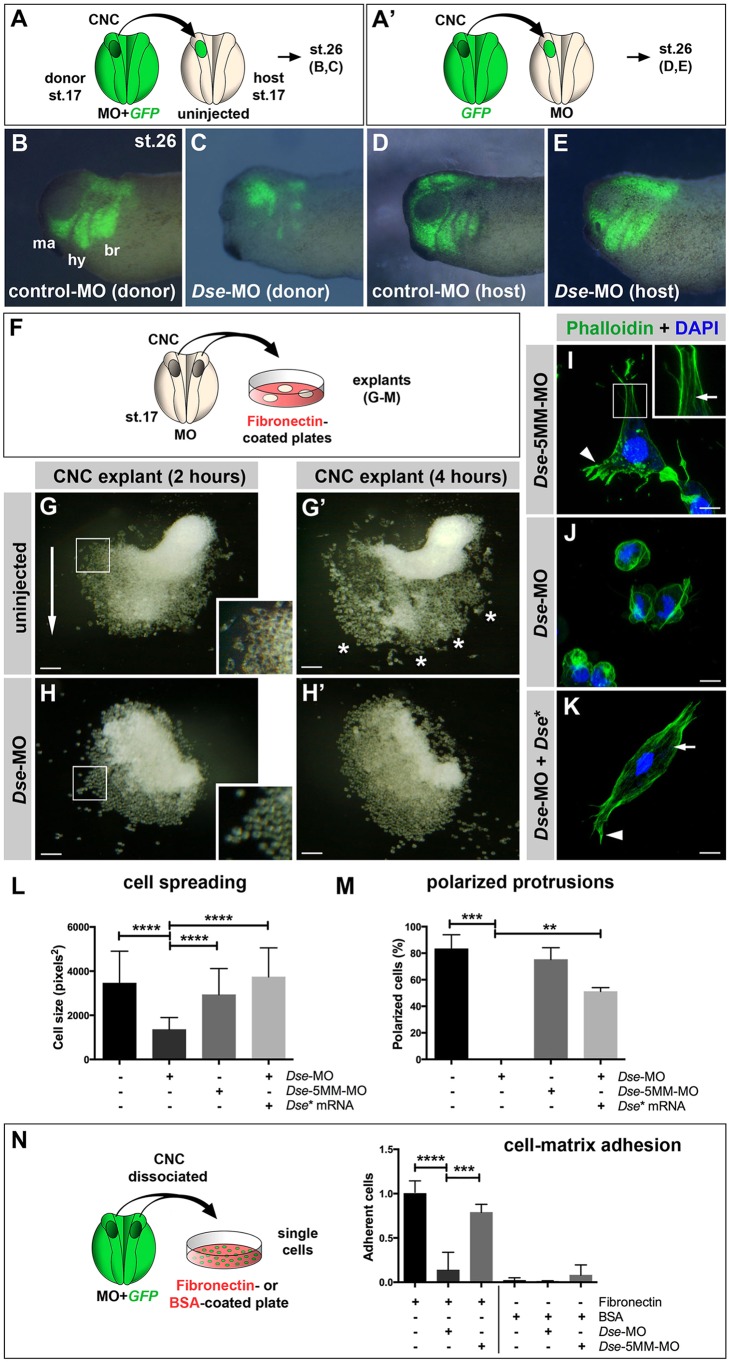
Fig. 6.
DS-epi1 has a tissue-autonomous role in CNC cell migration, adherence to fibronectin and cell polarization. (A,A′) Schemes for transplantation experiments. A CNC explant from an embryo injected with 300 pg GFP mRNA was homotypically grafted at stage 17. MOs were injected into the donor (A) or host embryo (A′). (B-E) Lateral view of embryos at stage 26. Grafted GFP+ CNC cells migrate ventrally when derived from control-MO-injected embryos (B); however, they do not properly migrate when derived from Dse-MO-injected embryos (C). br, branchial segment; hy, hyoid segment; ma, mandibular segment. The CNC cell migration was normal when the host embryo was injected with control-MO or Dse-MO (D,E). Three independent experiments were performed (n=3). (F) Scheme illustrating the culture of stage 17 morphant CNC explants on fibronectin-coated plates. (G,G′) At 2 h after plating (G), the control-MO-injected CNC explant exhibits collective cell migration in one direction (arrow). The inset shows a magnification of spread cells. After 4 h (G′), the cells migrate in distinct streams (asterisks). (H,H′) Cells of Dse-MO-injected CNC explants detach from each other and fail to adhere to the fibronectin substrate. The inset depicts a magnification of the spherical cells. (I-K) Confocal microscopy of fixed CNC cells after 5 h of explant culture on fibronectin. Phalloidin–Alexa-Fluor-488 and DAPI label F-actin and cell nuclei, respectively. The Dse-5MM-MO-injected control cell (I) exhibits lamellipodia at the leading edge (arrowhead) and stress fibers in the inner regions of the cell (arrow in inset). Dse-morphant cells (J) exhibit cortical networks of stress fibers and lack polarized protrusions. Co-injection of Dse-MO and 1 ng Dse* mRNA per embryo (K) restores the normal cytoskeleton and cell shape. (L,M) Quantification of cell spreading (L) and formation of polarized cell protrusions (M) in dissociated phalloidin-stained single cells from CNC explants following 5 h of culture on fibronectin. Cell spreading and polarized protrusions were quantified by calculating the cell size as the square number of pixels (ImageJ) and determining the percentage of cells with lamellipodia or filopodia, respectively. Uninjected and Dse-5MM-MO-injected explants exhibit a similar extent of cell spreading and formation of polarized protrusions. The reduction in the cell size and the lack of lamellipodia and filopodia are rescued by the co-injection of Dse* mRNA in Dse-morphant explants. A minimum of 100 cells per sample were evaluated in each experiment. Number of independent experiments (n≥3). Results are mean±s.d. (N) Cell–matrix adhesion of dissociated single CNC cells on fibronectin- or BSA-coated plates. Following the co-injection of MO and 300 pg GFP mRNA, CNC explants from stage 17 embryos were dissociated in Ca2+- and Mg2+-free medium and cultured for 45 min on fibronectin or BSA. The Dse-morphant cells exhibit decreased adhesion to fibronectin compared with the control and Dse-5MM-MO-injected cells. None of the analyzed cell samples exhibited significant cell adhesion to BSA. At least three independent experiments were performed for each sample (n≥3). Results are mean±s.d. The proportion of examined explants or cells with the indicated phenotype was as follows: B, 10/12; C, 11/13; D, 7/7; E, 9/9; G, 30/34; H, 26/28. Scale bars: 100 µm (G-H′); 10 µm (I-K). **P<0.01, ***P<0.001, ****P<0.0001 (one-way ANOVA multiple comparisons test with Tukey correction).