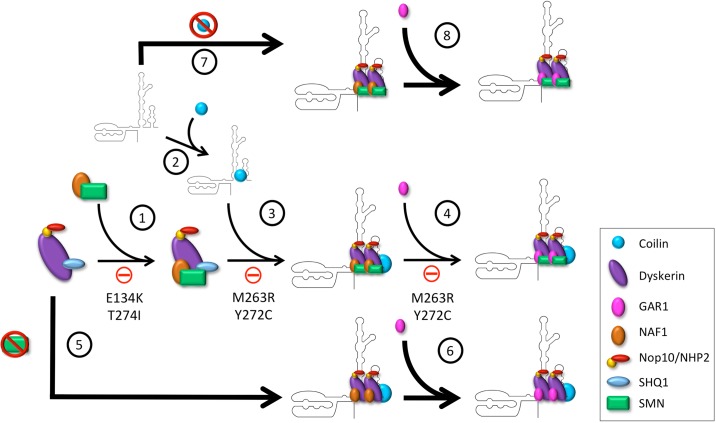
Fig. 7.
A model for the negative regulation of SMN and coilin on telomerase biogenesis. (1) NAF1 and SMN form a complex which is then loaded on to a pre-assembled dyskerin complex containing dyskerin, SHQ1, NHP2, and Nop10. However, the SMN mutations E134K and T274I inhibit this pathway. (2) Coilin binds to nascent hTR near dyskerin's binding site. (3) The dyskerin complex is then loaded onto hTR; however, coilin is not lost in this process and remains in the complex. SMN mutations M263R and Y272C inhibit the association of the dyskerin complex with hTR. (4) GAR1 is exchanged for NAF1 in the dyskerin complex. SMN mutations M263R and Y272C may also inhibit biogenesis at this step by blocking the association of GAR1 with the dyskerin complex. (5) If SMN expression is reduced, the dyskerin complex loads more readily on to hTR (indicated by a larger arrow), and (6) assembly of telomerase proceeds in the absence of SMN. (7) Similarly, if coilin expression is reduced, the dyskerin complex containing SMN will more readily bind hTR (indicated by a larger arrow), (8) and assembly will continue in the absence of coilin. Note: the stoichiometry shown here in regards to SMN and coilin in the telomerase complex is not intended to be definitive. In fact, interactions between SMN and coilin with telomerase components are most likely transient and require sub-stoichiometric amounts.