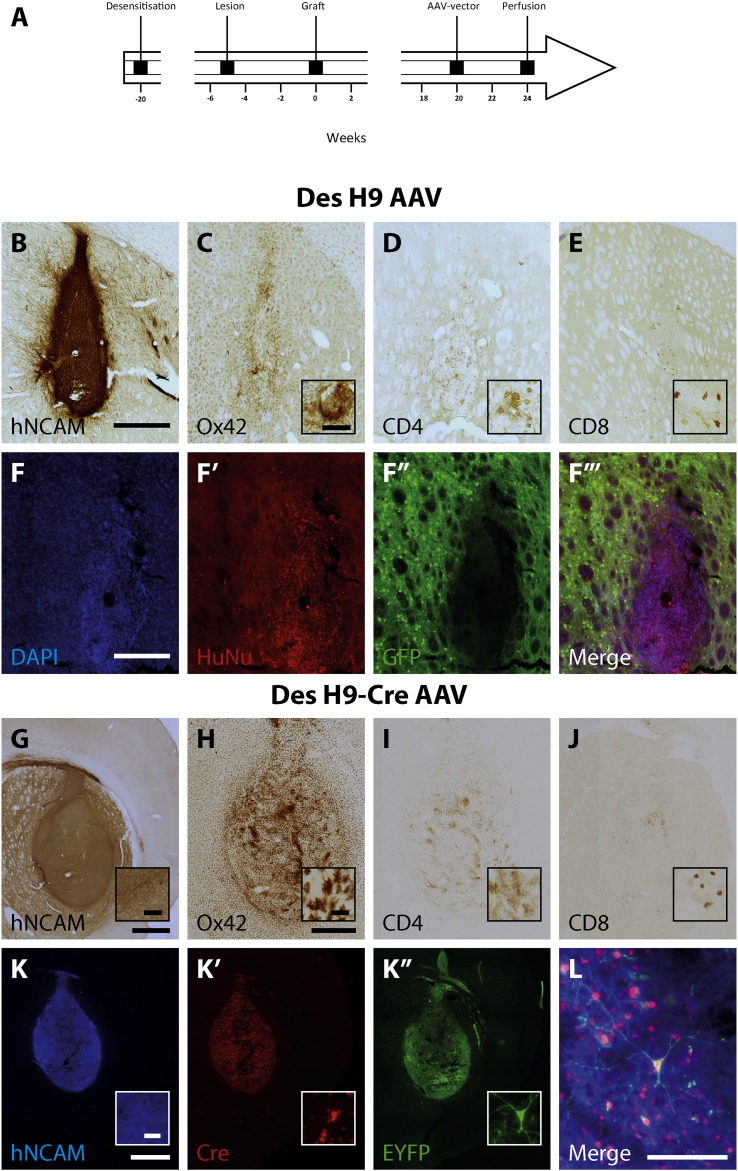
Fig. 4.
Characterisation of reopening the Blood Brain Barrier.
Rats that were neonatally desensitised using predifferentiated H9-hESCs and subsequently engrafted in adulthood using either H9-hESCs (Des H9 AAV; n = 4; A–F‴) or Cre-recombinase expressing H9-hESCs (Des H9-Cre AAV; n = 6; G–L) were subjected to a third opening of the blood brain barrier (after lesion and transplantation) to assess whether this would trigger an immune response causing the rejection of the transplant (A). We either used a GFP labelled AAV-vector to infect the host tissue (B–F‴) or a CRE-activatable direction inverse orientation (DIO) AAV-vector with a cassette encoding for EYFP to selectively infect the Cre-recombinase expressing donor cells (G–L) to select the cell population of interest. Transplants of both groups do survive long-term (> 24 weeks) in the rodent brain (B, G) and display limited inflammation (microglia; Ox42: C, H) and T-cell responses (CD4: D, I; CD8: E, J). Immune responses do not cause a rejection of the transplant within the four weeks after the respective AAV-transduction. Infection of the host cells was visualized using immune-fluorescent antibodies against DAPI (F) human nuclei (HuNU: F′), and GFP (F″). The composite merge shows the selective expression of the reporter in the host tissue (E‴). Selective transgene expression in the donor cells is demonstrated using fluorescent antibodies against human NCAM (J), Cre-recombinase (K′) and eYFP (K″). The composite merge (L) shows a cell that selectively expressed the EYFP reporter, clearly demonstrating the expression of nuclear Cre-recombinase and expression of EYFP. Scalebars: B–E = 500 μM; insets 50 μM; F–F‴ = 250 μM; G = 1000 μM, inset 50 μM; H–J = 1000 μM, inset 25 μM, K–K″ = 1000 μM, inset = 20 μM; L = 100 μM.