Table 1.
Activity of 1-methyl-5-nitroimidazole carboxamides and 4(5)-nitroimidazole carboxamides against G. lamblia, E. histolytica, T. vaginalis and C. difficile.
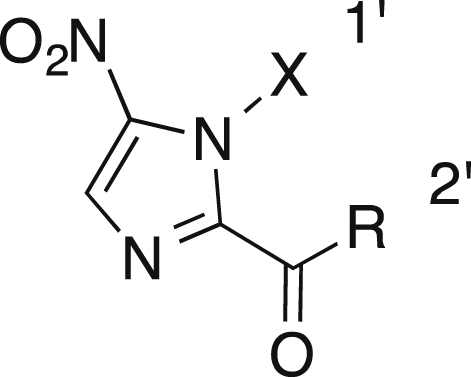 |
EC50 (μM) (pEC50 ± SE) |
MIC (μg/mL) |
CC50 (μM) (pCC50 ± SE) |
S.Ia (CC50/EC50) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
G. lamblia |
E. histolytica |
T. vaginalis |
C. difficileb |
HEK 293 |
Hep G2 |
|||||
| WB |
713M3 |
HM1:IMSS |
F1623 |
630 |
||||||
| No. | X | R | MtzS | MtzR | MtzS | MtzS | MtzS | |||
| 1 | Metronidazole | 6.1 (5.21 ± 0.05) | 18 (4.74 ± 0.02) | 5.0 (5.30 ± 0.03) | 0.8 (6.1 ± 0.07) | 0.5 | >100 | >100 | >16 | |
| 8a | Me | NHCH2(4-F-Ph) | 3.5 (5.46 ± 0.03) | 13 (4.89 ± 0.11) | 9.7 (5.01 ± 0.01) | 5.2 (5.28 ± 0.11) | >64 | >100 | >100 | >29 |
| 8b | Me | NHCH2(4-OCF3-Ph) | 3.0 (5.52 ± 0.03) | 8.8 (5.06 ± 0.29) | 14 (4.85 ± 0.02) | 13 (4.88 ± 0.01) | 32 | >100 | >100 | >33 |
| 8c | Me | NHCH2(3-OCF3-Ph) | 3.1 (5.51 ± 0.02) | 1.5 (5.84 ± 0.69) | 17 (4.77 ± 0.02) | 8.1 (5.09 ± 0.09) | 32–64 | >100 | >100 | >32 |
| 8d | Me | NHCHMe(4-F-Ph) | 2.9 (5.54 ± 0.03) | 1.9 (5.73 ± 0.39) | 10 (5.00 ± 0.03) | 3.8 (5.42 ± 0.24) | 64 | >100 | >100 | >34 |
| 8e | Me | NHCH2CH2(4-Me-Ph) | 2.8 (5.55 ± 0.03) | >20 (<4.70) | 13 (4.89 ± 0.03) | 8.1 (5.09 ± 0.16) | >64 | >100 | >100 | >36 |
| 8f | Me | NHCH2(2-pyridinyl) | 1.6 (5.80 ± 0.03) | 4.1 (5.39 ± 0.29) | 14 (4.85 ± 0.14) | 1.4 (5.84 ± 0.09) | 64 | >100 | >100 | >63 |
| 8g | Me | N(Me)2 | 2.4 (5.62 ± 0.03) | >20 (<4.70) | 14 (4.85 ± 0.03) | 1.3 (5.89 ± 0.30) | 32 | >100 | >100 | >42 |
| 8h | Me | morpholine | 1.6 (5.80 ± 0.03) | 11 (4.95 ± 0.20) | 14 (4.85 ± 0.02) | 0.6 (6.24 ± 0.34) | 32 | >100 | >100 | >63 |
| 8i | Me | pyrrolidine | 2.9 (5.54 ± 0.04) | 4.0 (5.40 ± 0.33) | 22 (4.66 ± 0.02) | 1.7 (5.76 ± 0.17) | 64 | >100 | >100 | >34 |
| 8j | Me | NH-cyclopropyl | 3.4 (5.47 ± 0.04) | 8.6 (5.06 ± 0.23) | 12 (4.92 ± 0.03) | 1.1 (5.96 ± 0.30) | >64 | >100 | >100 | >29 |
| 8k | Me | NH-cyclohexyl | 4.9 (5.31 ± 0.04) | 5.1 (5.30 ± 0.20) | 3.7 (5.43 ± 0.03) | 11 (4.97 ± 0.14) | >64 | >100 | >100 | >20 |
| 12a | H | NHCH2(4-F-Ph) | 0.5 (6.28 ± 0.10) | 2.4 (5.63 ± 0.35) | 3.6 (5.44 ± 0.06) | 2.3 (5.63 ± 0.17) | 4 | >100 | >100 | >250 |
| 12b | H | NHCH2(4-OCF3-Ph) | 0.2 (6.61 ± 0.03) | 2.5 (5.60 ± 0.38) | 4.5 (5.35 ± 0.06) | 2.2 (5.65 ± 0.23) | 16 | >100 | 93 (4.03 ± 0.07) | >500/465 |
| 12c | H | NHCH2(3-OCF3-Ph) | 0.2 (6.70 ± 0.03) | 15 (4.83 ± 0.11) | 3.6 (5.44 ± 0.01) | 1.9 (5.72 ± 0.17) | 16 | >100 | >100 | >500 |
| 12d | H | NHCHMe(4-F-Ph) | 0.4 (6.40 ± 0.6) | 1.3 (5.90 ± 0.31) | 1.7 (5.77 ± 0.04) | 0.6 (6.23 ± 0.14) | 8 | >100 | >100 | >250 |
| 12e | H | NHCH2CH2(4-Me-Ph) | 0.1 (7.00 ± 0.04) | 1.4 (5.87 ± 0.39) | 2.1 (5.68 ± 0.04) | 1.2 (5.92 ± 0.16) | 8 | >100 | >100 | >1000 |
| 12f | H | NHCH2(2-pyridinyl) | 7.2 (5.14 ± 0.03) | >20 (<4.70) | 15 (4.82 ± 0.02) | 7.1 (5.15 ± 0.11) | 2 | >100 | >100 | >14 |
| 12g | H | N(Me)2 | 8.3 (5.08 ± 0.02) | >20 (<4.70) | 2.7 (5.57 ± 0.03) | 2.4 (5.63 ± 0.11) | 2 | >100 | >100 | >12 |
| 12h | H | morpholine | 8.8 (5.06 ± 0.02) | >20 (<4.70) | 5.1 (5.29 ± 0.02) | 2.9 (5.54 ± 0.05) | 2 | >100 | >100 | >11 |
| 12i | H | pyrrolidine | 3.4 (5.47 ± 0.02) | 13 (4.88 ± 0.11) | 4.3 (5.37 ± 0.02) | 1.7 (5.77 ± 0.11) | 2 | >100 | >100 | >29 |
| 12j | H | NH-cyclopropyl | 5.0 (5.30 ± 0.03) | >20 (<4.70) | 5.3 (5.28 ± 0.02) | 4.4 (5.36 ± 0.05) | 1 | >100 | >100 | >20 |
| 12k | H | NH-cyclohexyl | 0.6 (6.22 ± 0.03) | 5.5 (5.26 ± 0.26) | 2.8 (5.55 ± 0.04) | 2.4 (5.61 ± 0.10) | 8 | >100 | >100 | >167 |
| 12l | H | NH2 | >50 (<4.3) | >20 (<4.70) | 13 (4.88 ± 0.03) | >20 (<4.70) | 1 | >100 | >100 | N/A |
| 12m | H | NHMe | 9.9 (5.00 ± 0.02) | >20 (<4.70) | 6.1 (5.21 ± 0.02) | 8.5 (5.07 ± 0.15) | 0.5–1 | >100 | >100 | >10 |
| 12n | H | NHCH2CH2OH | >50 (<4.3) | >20 (<4.70) | >50 (<4.3) | >20 (<4.70) | 8 | >100 | >100 | N/A |
| 12o | H | NMeCH2CH2OH | 24 (4.62 ± 0.05) | >20 (<4.70) | >50 (<4.3) | >20 (<4.70) | 2 | >100 | >100 | >4 |
Selectivity Index: average cytotoxicity against HEK293 and HepG2 cell lines/G. lamblia WB activity(CC50/EC50).
Similar results were obtained for C. difficile NAP/027 strain (Supplementary Table 3). EC50 minimum n = 3 EC50, pEC50 ± SE, MIC median of n = 4, CC50 n = 3, pCC50 ± SE.
