Table 2.
In vitro activity of 1-substituted 4-nitroimidazoles against G. lamblia and E. histolytica.
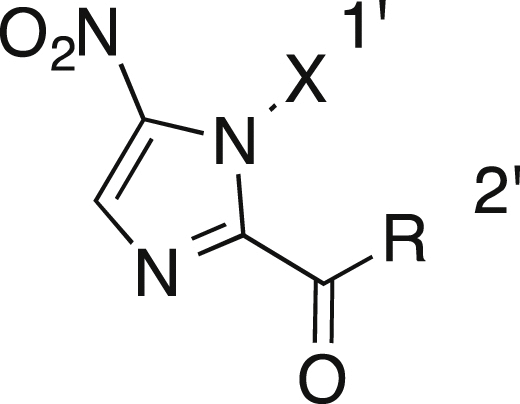 |
EC50 (μM) (pEC50 ± SE) |
MIC (μg/mL) |
CC50 (μM) (pCC50 ± SE) |
S.Ia (CC50/EC50) | ||||
|---|---|---|---|---|---|---|---|---|
|
G. lamblia |
E. histolytica |
C. difficile |
HEK293 | HepG2 | ||||
| No. | X | R | WB | HM1:IMSS | 630 | |||
| 1 | Metronidazole | 6.1 (5.21 ± 0.05) | 5.0 (5.30 ± 0.03) | 0.5 | >100 | >100 | >16 | |
| 13a | CH2(4-F-Ph) | -NH2 | >50 (<4.3) | >50 (<4.3) | >64 | >100 | >100 | N/A |
| 13b | CH2(4-OCF3-Ph) | -NH2 | 4.1 (5.39 ± 0.03) | >25 (<4.6) | >64 | >100 | >100 | >22 |
| 13c | CH2(3-OCF3-Ph) | -NH2 | 8.4 (5.08 ± 0.05) | >50 (<4.3) | >64 | >100 | >100 | >12 |
| 13d | CH2CH2(4-Me-Ph) | -NH2 | >50 (<4.3) | >50 (<4.3) | >64 | >100 | >100 | N/A |
| 13e | CH2(2-pyridinyl) | -NH2 | 27 (4.57 ± 0.07) | >50 (<4.3) | >64 | >100 | >100 | >3.7 |
| 13f | CH2-cyclopropyl | -NH2 | >50 (<4.3) | >50 (<4.3) | >64 | >100 | >100 | N/A |
| 13g | CH2-cyclohexyl | -NH2 | 5.0 (5.30 ± 0.06) | >50 (<4.3) | >64 | >100 | >100 | >20 |
| 14a | CH2(4-OCF3-Ph) | -NHMe | 3.4 (5.47 ± 0.04) | 30 (4.52 ± 0.03) | 64–>64 | >100 | >100 | >29 |
| 14b | CH2(4-OCF3-Ph) | -NMe2 | 2.7 (5.57 ± 0.04) | 19 (4.72 ± 0.04) | 64 | >100 | >100 | >37 |
| 14c | CH2(4-OCF3-Ph) | -OEt | 6.0 (5.22 ± 0.05) | 51 (4.29 ± 0.03) | >64 | >100 | >100 | >17 |
| 14d | CH2(4-OCF3-Ph) | -NHOH | 5.1 (5.29 ± 0.04) | 10 (5.00 ± 0.03) | 16 | 36 (4.44 ± 0.06) | >100 | 7/>20 |
| 14e | CH2(4-OCF3-Ph) | -NHNH2 | 7.5 (5.12 ± 0.05) | 45 (4.35 ± 0.03) | >64 | >100 | >100 | >13 |
Selectivity Index: average cytotoxicity of HEK293 and HepG2 cell lines/G. lamblia WB activity (CC50/EC50). EC50 minimum n = 3 EC50 (pEC50 ± SE), MIC median of n = 4, CC50 n = 3 (pCC50 ± SE).
