Abstract
Background
Autophagy serves as a cellular protective mechanism against alcohol induced tissue injury but excessive autophagy can also be detrimental leading to apoptosis. Our lab has previously shown that moderate alcohol consumption alters expression of proteins in the insulin signaling pathway and worsens glucose metabolism in the liver in a swine model of metabolic syndrome. We examined the effect of alcohol consumption on apoptosis and autophagy signaling in the liver in our clinically relevant animal model of chronic hypercholesterolemia.
Materials
Twenty-six Yorkshire swine were fed a high fat diet for 4 weeks then split into 3 groups: hypercholesterolemic diet alone (HCC, n= 9), hypercholesterolemic diet with vodka (HCV, n= 9), and hypercholesterolemic diet with wine (HCW, n = 8) for 7 weeks. Animals underwent euthanasia and liver tissue samples were harvested for analysis.
Methods
Liver tissue was analyzed via western blot analysis. Protein density data was normalized to GAPDH and is reported as fold change values+/− SEM compared to the high cholesterol diet control group. A Kruskal Wallis test with a Dunn's multiple comparison test was used to compare the means among groups.
Results
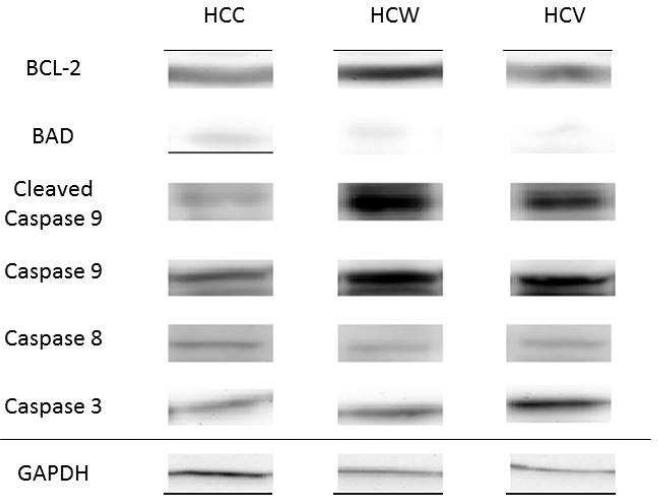
The HCVgroup showed significant increases in several pro apoptotic proteins (including caspase 3, caspase 8, caspase 9 and cleaved caspase 9) compared to the HCC group. There was a decrease in the pro apoptotic protein (BAD) and an increase in anti-apoptotic signal (BCL-2) in the HCW group compared to HCC control. There were increases in pro-survival proteins (AKT, p-AKT, mTOR, p-mTOR) in the HCW and the HCV group compared to control (HCC). There were decreases in autophagy protein LCB-3 in the HCW and HCV compared to the control.
Conclusions
We found that moderate alcohol consumption altered protein expression related to apoptosis and autophagy signaling in pig liver in the setting of hypercholesterolemia. Interestingly, vodka may induce pro-apoptotic pathways in liver tissue, whereas wine may induce anti apoptotic signaling. These results provide a mechanism by which vodka may contribute to alcoholic liver disease and supports the notion that wine, containing resveratrol, may prevent cellular apoptosis in liver tissue in the setting of hypercholesterolemia.
Introduction
Obesity in the United States is a serious health problem. Obesity is associated with multiple comorbid conditions including hypercholesterolemia, hypertriglyceridemia, high blood pressure, glucose intolerance leading to type 2 diabetes mellitus. These comorbidities have been described by the term metabolic syndrome. [1,2] Approximately 50 million Americans are affected by metabolic syndrome and these patients have an increased risk of mortality from cardiovascular disease. [1,2] Alcohol consumption is one of the modifiable risk factors for the development of diabetes and cardiovascular disease.[3].
Moderate consumption of alcohol is thought to be beneficial to human health however excessive consumption (in either a binge or chronic form) is thought to be detrimental to human health. [4,5] Moderate alcohol consumption is defined as 1-2 drinks/per day (or 20-30g alcohol/day) while chronic or binge consumption is described as (3-5 drinks/day or 6+drinks per day or >60g alcohol/day, respectively). [3,5,6] Autophagy serves as a protective mechanism against alcohol induced tissue injury but can also be detrimental leading to cellular apoptosis. [7] Acute alcohol (or binge) administration (EtOH at doses of 3.0-7.0 g/kg.) increases autophagy in cultured murine hepatocytes and in mouse livers. [4,8] In contrast chronic alcohol consumption has been proven to lead to protein accumulation and hepatomegaly in the liver and may be associated with impaired autophagy in liver tissue. [7,9] This inhibition of autophagy exacerbates alcohol induced cell death and liver injury [9] It is thought that autophagy protects against alcohol induced liver injury by preventing cellular apoptosis. Interestingly, the effect of moderate alcohol consumption (5-25g/day) in the liver is largely unstudied even though it has been found to be beneficial to the heart. [9–12]
Resveratrol is a poly-phenol which is extracted from red grapes, among other plants, and can be found in red wine. Research suggests that resveratrol is the heart healthy component of red wine. Resveratrol has also been shown to: 1) aid in the transport of cholesterol from peripheral tissues to the liver which helps to improve the abnormal lipid metabolism associated with type 2 diabetes mellitus and 2) to improve insulin sensitivity and signaling in the liver. [2,13,14] Our lab has previously shown that resveratrol improves the weight gain, obesity and insulin resistance induced by a high-fat diet used in a swine model of metabolic syndrome. In this model, resveratrol modulates proteins involved in insulin signaling in the liver tissue.[2] We then examined if consumption of standard alcoholic beverages (that do and do not contain common amounts of resveratrol (red wine and vodka) provides similar effects to resveratrol alone. Interestingly, we found that moderate consumption of standard alcoholic beverages worsens glucose metabolism by modulating the expression of proteins involved in insulin signaling pathways in the liver. [3]
The first stage of chronic alcoholic liver disease is hepatic steatosis. Hepatic steatosis is a caused by lipotoxicity, inflammation, lipid accumulation, oxidative stress and impaired lipid metabolism.[15] Interestingly, it is thought that certain concentrations of alcohol may protect against hepatic disease by promoting cellular autophagy. [16] Resveratrol is believed to have therapeutic effects on hepatic steatosis by regulating autophagic signaling pathways. [15]
In this study, we examined the effect of moderate alcohol consumption on apoptosis and autophagy signaling in the liver in our clinically relevant animal model of chronic hypercholesterolemia.
Materials and Methods
Animal Model
Experiments were performed as previously described. [2] Twenty-six Yorkshire swine (Parsons Research, Amherst, MA) were fed a high cholesterol diet (500g/day) (Sinclair Research, Columbia, MO) for 4 weeks after which time an ameroid constrictor was placed on the left circumflex artery. [3] At week 4, the animals were placed into 3 groups: the control group was continued on a hypercholesterolemic diet alone (HCC) (n=9); the hypercholesterolemic vodka (HCV) group was given 112 mL of vodka per day (40% EtOH/V, Rubinoff Vodka, Somerville, MA) (n=9) and the hypercholesterolemic wine (HCW) group was given 375 mL of red wine per day (12.5%EtOH/V, 2009 Pinot Noir, Black Mountain Vineyard, Napa and Sonoma, CA) (n=8) .[2]. The amount of ethanol was similar between groups (HCC-45mls ethanol/day, HCW-47mls ethanol/day). The high fat diet used in our animal model administered for four weeks has been showed to induce obesity, hyperlipidemia, high blood pressure, insulin resistance and glucose intolerance. [17] The average weight for the pigs was 18.2 kg at the first procedure and 35.3kg at the second procedure. The pigs were fasted overnight prior to the day of each procedure. Although fasting may affect some of the signaling cascades studied, all groups including the control were treated similarly and therefore we do not feel this would appreciably change our finding regarding relative differences in protein expression with alcohol consumption.
After 7 weeks all animals were euthanized by exsanguination and liver muscle samples were collected. Liquid nitrogen was used to freeze tissue samples. Experiments using these animals have established that 7 weeks is enough time to see differences in myocardial perfusion and angiogenic and insulin signaling pathways. This data has been previously published. [3,18] Experiments were reviewed and approved by the Rhode Island Hospital Institutional Animal Care and Use Committee. The “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” was used to ensure adequate care of all animals.
Protein Expression
Tissue lysates and western blots were performed as previously described. [19] Liver tissue was lysed in radio-immuno-precipitation assay buffer (RIPA), separated using Sodium dodecyl sulfide polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene diflouride membranes (PVDF). The membranes were then incubated with primary antibodies overnight at 4 degrees C against caspase 3, caspase 8, caspase 9, cleaved caspase, Bad, BCL-2, AKT, p-AKT, mTOR, p-mTOR, LCB-3, p-BCL-2, AMPKα, TNF-α, Beclin 1, Lamp-1, Lamp-2, cleaved caspase 3, Bax, p-Bad, LKB-1, p-AMPKα, and ATG5 (Cell Signaling, Danvers, MA). To correct for loading error, all membranes were probed with GAPDH (Cell Signaling, Danvers, MA). Membranes were incubated at room temperature with a horseradish peroxidase-linked secondary antibody for 1h (Jackson ImmunoResearch, West Grove, PA). Chemiluminescence was used to visualize immune complexes. A digital camera system was used to take a photo of the images (G-Box, Syngene, and Cambridge, England). Band densitometry was measured using Image J Software.
Data Analysis
Protein density data is reported as fold change values +/− SEM compared to the hypercholesterolemic control group. GraphPad Prism 5.0 Software was used to compare the mean fold change among the three groups by a Kruskal Wallis test with a Dunn's multiple comparison post-hoc test. (GraphPad Software Inc, Sand Diego, Ca). Prior to analyzing protein rations (activated to total protein forms) each protein was normalized to GAPDH or α-tubulin to control for loading error.
Results
Alcohol Increases ProApoptotic Protein Signaling
There was a significant decrease in expression of the pro-apoptotic protein BAD in the hypercholesterolemic wine and hypercholesterolemic vodka groups compared to control. However, when comparing the ratio of p-BAD to BAD there was no significant change between the groups. [Figure 1, 2A] There were significant increases in the pro-apoptotic proteins caspase 3, caspase 8, caspase 9 and cleaved caspase 9 in the hypercholesterolemic vodka group compared to control [Figure 1, 2A-D]. There was a nonsignificant decrease in cleaved caspase 3 in the hypercholesterolemic wine and hypercholesterolemic vodka group compared to the control. When comparing the ratio of cleaved caspase 3 to caspase 3 there was a significant decrease in the hypercholesterolemic vodka group compared to the control group. [Figure 1, 2B] Similarly, when comparing cleaved caspase 9 to caspase 9 there was a significant decrease in the hypercholesterolemic vodka group compared to the control group. [Figure 1, 2C]
Figure 1. Alcohol Increases Proteins Involved in Apoptotic Pathways.
Representative images of apoptotic proteins in HCC, HCW and HCV groups. GAPDH shows representative images for loading control.
Figure 2[A-F]. Alcohol Increases Proteins Involved in Apoptotic Pathways.
Quantitative changes in apoptotic signaling proteins are demonstrated in bar diagrams. A,B,C,E: Include quantitative data demonstrating ratio of activated to total protein HCC- hypercholesterolemic control, HCW- hypercholesterolemic wine group, HVC- hypercholesterolemic vodka group *= p<0.05 by a Kruskal Wallis test with a Dunn's multiple comparison test.
There was a significant increase in anti-apoptotic signal BCL-2 in the hypercholesterolemic wine group compared to the hypercholesterolemic vodka group and a nonsignificant increase in the hypercholesterolemic wine group compared to control. There was a nonsignificant increase in p-BCL-2 in the hypercholesterolemic wine and hypercholesterolemic vodka groups compared to the control group. When comparing the ratio of p-BCL-2 to BCL-2 there was a trend for increase in both the hypercholesterolemic wine and vodka groups compared to the control (ANOVA on ranks. p<0.05) however this did not reach significance with the post hoc Dunn's test. [Figure 1, 2E]
There were no significant differences from control in the hypercholesterolemic wine and hypercholesterolemic vodka groups in Bax, compared to the control. [Figure 2F]
Alcohol and Wine Modulate Proteins Involved in Autophagy
There were significant increases in the pro-survival proteins AKT and p-AKT in the hypercholesterolemic wine group and the hypercholesterolemic vodka group compared to control. When comparing p-AKT to AKT there was no significant change between the groups. [Figure 3, 4A] There was a significant increase in the downstream effector mTOR in hypercholesterolemic wine group compared to hypercholesterolemic vodka group and a nonsignificant increase compared to control. There was a significant increase in p-mTOR in the hypercholesterolemic vodka group compared to the control. When comparing the ratios of pmTOR to mTOR there was a significant increase in the hypercholesterolemic vodka group compared to the control group and compared to the hypercholesterolemic wine group. [Figure 3, 4B] There was a non-significant increase in the hypercholesterolemic vodka group in AMPKα expression compared to control. However there was a nonsignficant decrease in p-AMPKα compared to the control. p-AMPKA to AMPKA There was a nonsignficant decrease in the ratio of p-AMPKα AMPKa hypercholesterolemic vodka group compared to the control group. [Figure 4C].
Figure 3. Alcohol and Wine Modulate Proteins Involved in Autophagy Pathways.
Representative images of autophagy proteins in HCC, HCW and HCV groups. GAPDH shows representative images for loading control.
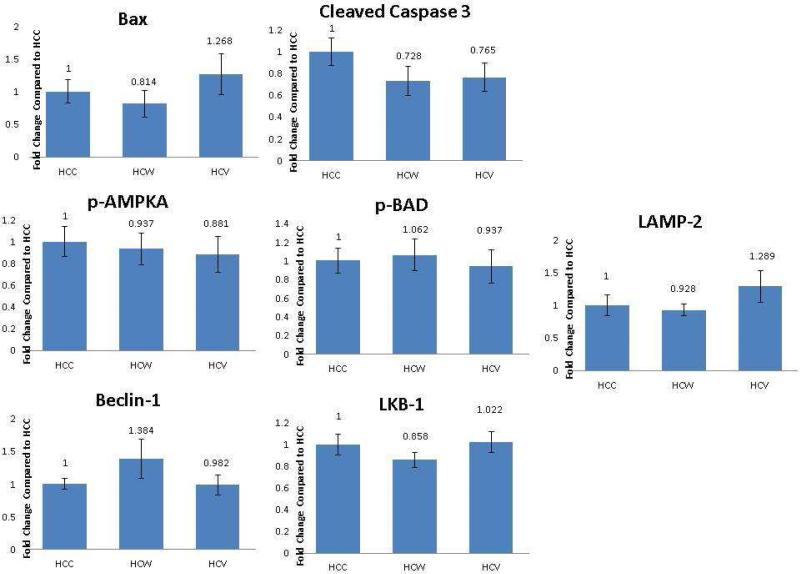
Figure 4 [A-J]. Alcohol and Wine Modulate Proteins Involved in Autophagy Pathways.
Quantitative changes in autophagy Signaling Proteins are demonstrated in bar diagrams. A,B,C: Include quantitative data demonstrating ratio of activated to total protein HCC- hypercholesterolemic control, HCW- hypercholesterolemic wine group, HVC- hypercholesterolemic vodka group *= p<0.05 a Kruskal Wallis test with a Dunn's multiple comparison test.
There was a significant decrease in LCB-3 in the hypercholesterolemic wine group compared to the control and nonsignificant decrease in the hypercholesterolemic vodka group compared to the control. [Figure 4D] There was a nonsignificant increase in the hypercholesterolemic vodka group in TNF-α compared to control. [Figure 4E] There were non-significant increases in in pro-autophagy protein Lamp-1 in the hypercholesterolemic wine group and non-significant decrease in the hypercholesterolemic vodka group compared to control. [Figure 4F]
There was a nonsignificant increase in Beclin-1 in the hypercholesterolemic wine group compared to the control [Figure 4G] and a nonsignficant increase in LAMP-2 compared to the control. [Figure 4H] There was a no change in LKB-1 between groups. [Figure 4I] In ATG5 there were nonsignficant decreases in the hypercholesterolemic wine and hypercholesterolemic vodka groups compared to the control. [Figure 4J]
Discussion
In this study we found that moderate alcohol consumption alters apoptosis and autophagy signaling in the liver in a clinically relevant animal model of chronic myocardial ischemia and hypercholesterolemia. We found that moderate alcohol and wine consumption altered cell survival protein expression related to apoptosis and autophagy in pig liver in the setting of hypercholesterolemia. Interestingly, vodka may induce pro-apoptotic pathways in liver tissue, whereas wine may induce anti apoptotic signaling.
As we previously reported, the hypercholesterolemic vodka, hypercholesterolemic wine and hypercholesterolemic control groups had similar liver function tests and H&E staining of liver sections in each of the groups indicated no evidence of overt hepatocellular injury. [3] This data suggests that the neither moderate vodka nor wine consumption lead to liver damage in the pigs used in this study.
Caspases are intracellular proteases that work during apoptosis to disassemble the cell into apoptotic bodies. Initiator caspases, including caspase-8 and caspase-9, activate executioner caspases, including caspase 3, which are responsible for carrying out final steps in the disassembling process. Elevated levels of active forms of the caspases are considered markers of cellular apoptosis. [20] We found that there were significant increases in the expression of several of these apoptotic markers including caspase 3, caspase 8, caspase 9 and cleaved caspase 9 in the hypercholesterolemic vodka group compared to control. While we saw an increase in total caspase 3 in the hypercholesterolemic vodka group compared to control there was no change in the cleaved caspase 3. However, owing to the large increase in caspase 3 expression, the ratio of cleaved caspase 3 to caspase3 decreased significantly in the hypercholesterolemic vodka group. Future research will need to be performed to determine the effect these chronic changes in caspase 3 total expression are having on apoptosis and if there are any induced regulatory pathways that can explain the normalized levels of cleaved caspase 3 . Interestingly, we saw an increase in both caspase 9 and cleaved caspase 9 in the hypercholesterolemic vodka group compared to the control. There was no difference in ratio of cleaved to total caspase 9 between groups but this is likely do to the fact that both the total and activate cleaved form were increased in equal proportions.
This data is consistent with previous research that showed in alcoholic liver disease alcohol stimulates macrophage activation through caspase dependent release of extracellular vesicles. The content of these vesicles then contribute to inflammation. [21] Importantly, wine did not increase expression of pro apoptotic signaling pathways. This is consistent with the fact that resveratrol has been found to have a protective effect in liver disease [2, 13–15]. This difference in the effect of vodka and wine on apoptotic signaling is one interesting finding of our study. Future work will need to look at this pathway more thoroughly and explore its association with inflammatory cells and cell death in the pig liver tissue.
B-cell lymphoma-2 (BCL-2) family members regulate apoptosis and cell-cycle regulation. BCL-2 is anti-apoptotic and anti-proliferative. It functions by facilitating the cell cycle into the G0 phase. BAX is pro-apoptotic and functions by accelerating the cell cycle into the S-phase. BAD is pro-apoptotic and functions by inhibiting both cell cycle progression and the activity of BCL-2. [22] We found significant decreases in pro-apoptotic protein Bad in the hypercholesterolemic wine and hypercholesterolemic vodka groups compared to control. We found no significant change in p-BAD although we did see a nonsignificant increase in the p-BAD/BAD ratio in the hypercholesterolemic wine and hypercholesterolemic vodka group compared to the control. In these studies, it may be that BAD levels serves as a marker for decreased apoptotic cells in the hypercholesterolemic vodka and wine groups. However, the nonsignificant increase in the p-BAD/BAD ratio may indicate that active apoptosis occured to a greater extent in the hypercholesterolemic vodka and wine groups compared to the control. Future research will have to evaluate the abundance of apoptotic cells in the tissue directly.
Interestingly, the wine group had increases in anti-apoptotic signaling demonstrated by an increase in BCL-2. The vodka group did not show similar increases in anti apoptotic BCL-2. We saw no change between the groups in p-BCL-2 or in the ratio of pBCL-2/BCL. We also saw no significant difference between groups in Bax protein. This is in contrast to a recent study which found that resveratrol promotes cellular apoptosis in human leukemia K563 cells by increasing the expression of Bax compared to BCL-2. [23] Future work will be required to further investigate the anti-apoptotic pathway and to determine if enhanced anti apoptotic signaling in the wine group results in reduced liver injury.
The PI3K/AKT/AMPKα/mTOR signaling pathway regulates autophagy when cells are under stressful conditions. [24,25]. Recent research has found that hydrogen sulfide administration improves nonalcoholic fatty liver disease by activating the AMPKα/mTOR autophagy pathway. [26] Interestingly, resveratrol improves both the health and survival of mice on high calorie diets which was associated with up-regulation of the AMPKα pathway [13] We found that both vodka and wine significantly increases the expression of AKT and p-AKT in pig liver. There was no difference in ratio of p-AKT to AKT between groups but this is likely do to the fact that both the total and phosphorylated form were increased in equal proportions. Both vodka and wine also modulated the expression of downstream signals including mTOR and pmTOR. . Interestingly, there was a significant increase in total mTOR in the hypercholesterolemic wine group compared to the hypercholesterolemic vodka group. However, there was a significant increase in p-mTOR in the hypercholesterolemic vodka group compared to the control and an increase in the p-mTOR to total mTOR ratio in the hypercholesterolemic vodka group compared to both the hypercholesterolemic wine and control groups. Finally, we actually found that AMPKα was not significantly changed between our groups. However there was a non-significant increase in AMPKα expression and a decrease in p-AMPKα and the p-AMPKα/AMPKα ratio in the hypercholesterolemic vodka group compared to the control. This data may suggest a possible decreased activity of this protein. It is possible that the both alcohol and wine work to increase autophagy through these pathways.
Light Chain 3 (LC3) is known to be a marker for cellular autophagy. LC3 undergoes post translational modifications during autophagy including cleavage at the carboxy terminus creating a LC3 cytosolic isoform. The presence of total LC3, and its cleaved isoform, is used as an indicator of autophagy. [27] The lysosomal membrane proteins LAMP-1 and LAMP-2 are not only critical components of the lysosomal membrane but also serve a regulatory role in during cellular autophagy [28]. Members of the tumor necrosis factor (TNF) family are involved in maintenance of the immune system. However they have also been found to have a role in stimulation of both autophagy and apoptosis. We found that both vodka and wine modulate the expression of these known markers of autophagy, LCB, TNF, and LAMP-1, when compared to the control pigs. This is consistent with research that shows that resveratrol improves hepatic steatosis by inducing autophagy pathways. [15,29] Interestingly, the effects of vodka on autophagy are not well described. It appears that autophagy can protect hepatocytes from apoptosis associated with alcohol at certain concentrations. [16] Future work will be required to further investigate the relevance of autophagy to alcoholic induced liver disease.
Conclusions
While it has been well studied that acute and chronic alcohol consumption leads to alcohol liver disease and that resveratrol, a key component in wine, is protective against alcohol liver disease there is limited information regarding the effect of moderate alcohol consumption on liver tissue. Studies have shown that people who consume moderate quantities of alcohol/day (2 drinks or 20g/day) have a lower reduced risk of developing diabetes compared to abstainers from alcohol. In addition those who consume high quantities of alcohol (>5 drinks or >60g/day) have an increased risk of developing diabetes. The dose dependent effect of alcohol is not well understood and there may be a difference between different types of alcohol ingested and their effects on diabetes development. For example, certain concentrations of alcohol may protect against hepatic disease by promoting cellular autophagy [16] and resveratrol is believed to have therapeutic effects on hepatic steatosis by regulating autophagic signaling pathways. In this study, we found that moderate alcohol consumption alters apoptosis and autophagy signaling in the liver in a clinically relevant animal model of chronic myocardial ischemia and hypercholesterolemia. Interestingly, vodka may induce pro-apoptotic pathways in liver tissue, whereas wine may induce anti apoptotic signaling. These results provide a mechanism by which vodka may contribute to alcoholic liver disease and supports the notion that wine, containing resveratrol, may prevent cellular apoptosis in liver tissue in the setting of hypercholesterolemia.
Limitations
This swine model of metabolic syndrome closely approximates the comorbid conditions associated with obesity. However species differences are a concern given the fact that swine metabolize alcohol more rapidly than do humans. In addition, the long term effects of a high cholesterol diet may have been limited by the 11 week time frame of the study. Finally, the dose dependent effect of alcohol on liver function is not fully understood. [14,16] Further investigation in our swine model is warranted to determine the dose-response relationship between alcohol consumption, autophagy signaling and hepatic steatosis. Finally, only male animals were used in our study generating the possibility of gender bias in our results. Given this is a large animal model; we kept the number of animals to a minimal while still allowing the appropriate statistical power.
Table 1.
| Protein BCL-2 |
HCC | HCW | HCVOD | ANOVA Pvalue | Protein MTOR |
HCC | HCW | HCVOC | ANOVA Pvalue |
| 1+/− 0.18 | 1.33+/− 0.66 | 0.60+/− 0.25 | 0.0113 | 1+/− 0.37 | 2.35 +/− 1.35 | 0.77+/− 0.49 | 0.037 | ||
| Caspase 8 | 1+/− 1.23 | 0.74 +/− 0.438 | 2.37 +/− 2.58 | 0.019 | BAD | 1+/− 0.20 | 0.64 +/− 0.17 | 0.68+/− 0.21 | 0.009 |
| Caspase 3 | 1+/− 0.66 | 1.30+/− 1.09 | 3.37+/− 1.75 | 0.013 | p-AKT | 1+/− 0.21 | 1.82 +/− 0.48 | 2.0+/− 0.52 | 0.0009 |
| AKT | 1+/− 0.22 | 1.80+/− 0.34 | 3.18+/− 1.39 | 0.0002 | Cleaved Caspase 9 | 1+/− 0.24 | 1.47 +/− 0.84 | 1.47+/− 0.22 | 0.013 |
| P-MTOR | 1+.− 1.36 | 0.91+/− 0.46 | 2.21+/− 1.76 | 0.034 | Caspase 9 | 1+/− 0.27 | 1.21 +/− 0.27 | 1.39 +/− 0.19 | 0.03 |
| LCB-3 | 1+/− .22 | 0.54+/− 0.19 | 0.74+/− 0.28 | 0.01 | All numbers represent fold values +/− SD compared to HCC and normalized to GAPDH. Or alpha tubulin | ||||
Acknowledgments
This work was supported in part by the National Heart, Lung, and Blood Institute (R01HL46716, R01HL69024) to Dr. Sellke; NIH/NIGMS Training Grant 2T32 GM065085 to Dr. Potz;
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: BAP performed study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript and critical revision of manuscript. IJL performed acquisition of data, analysis and interpretation of data, critical revision of manuscript. RTC performed critical revision of manuscript. FWS study conception and design, drafting of manuscript and critical revision of manuscript
Disclosures: None
Presented: AAS Paper: Presented at the 11th Annual Academic Surgical Congress, Jacksonville, Florida, and February 2-4th 2016
References
- 1.Malik S. Impact of the Metabolic Syndrome on Mortality From Coronary Heart Disease, Cardiovascular Disease, and All Causes in United States Adults. Circulation. 2004;110:1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E. doi:10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 2.Burgess TA, Robich MP, Chu LM, Bianchi C, Sellke FW. Improving glucose metabolism with resveratrol in a swine model of metabolic syndrome through alteration of signaling pathways in the liver and skeletal muscle. Arch Surg. 2011;146:556–64. doi: 10.1001/archsurg.2011.100. doi:10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nassrene Y, Elmadhun MD1, Antonio D, Lassaletta MD1, Louis M, Chu, MD1 C, Bianchi MD, PhD1, Frank W, Sellke M. Vodka And Wine Consumption In A Swine Model Of Metabolic Syndrome Alters Insulin Signaling Pathways In The Liver And Skeletal Muscle. Surgery. 2012;152:414–22. doi: 10.1016/j.surg.2012.06.014. doi:10.1016/j.surg.2012.06.014.Vodka. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeevaart JG, Wang L, Thakur V V, Leung CS, Tirado J, Bailey CM, et al. Autophagy Reduces Acute Ethanol-Induced Hepatotoxicity and Steatosis in Mice. Gastroenterology. 2010;139:1740–52. doi: 10.1053/j.gastro.2010.07.041. doi:10.1021/ja8019214.Optimization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldfinger TM. Beyond the French paradox: the impact of moderate beverage alcohol and wine consumption in the prevention of cardiovascular disease. Cardiol Clin. 2003;21:449–57. doi: 10.1016/s0733-8651(03)00081-x. doi:10.1016/S0733-8651(03)00081-X. [DOI] [PubMed] [Google Scholar]
- 6.Klatsky a. L, Armstrong M a., Friedman GD. Alcohol and mortality. Ann Intern Med. 1992;117:646–54. doi: 10.7326/0003-4819-117-8-646. doi:10.1016/S0140-6736(81)92326-6. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Wang S, Ni H-M, Huang H, Ding W-X. Autophagy in alcohol-induced multiorgan injury: mechanisms and potential therapeutic targets. Biomed Res Int. 2014;2014:498491. doi: 10.1155/2014/498491. doi:10.1155/2014/498491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni HM, Du K, You M, Ding WX. Critical role of FoxO3a in alcohol-induced autophagy and hepatotoxicity. Am J Pathol. 2013;183:1815–25. doi: 10.1016/j.ajpath.2013.08.011. doi:10.1016/j.ajpath.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams JA, Ding W. A Mechanistic Review of Mitophagy and Its Role in Protection against Alcoholic Liver Disease. Biomolecules. 2015;5:2619–42. doi: 10.3390/biom5042619. doi:10.3390/biom5042619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lassaletta AD, Elmadhun NY, Liu Y, Feng J, Burgess T a, Karlson NW, et al. Ethanol promotes arteriogenesis and restores perfusion to chronically ischemic myocardium. Circulation. 2013;128:S136–43. doi: 10.1161/CIRCULATIONAHA.112.000207. doi:10.1161/CIRCULATIONAHA.112.000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmadhun NY, Sabe AA, Lassaletta AD, Sellke FW. Ethanol promotes new vessel growth in remote nonischemic myocardium. J Surg Res. 2015;193:536–42. doi: 10.1016/j.jss.2014.05.048. doi:10.1016/j.jss.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Alcohol consumption and mortality in patients with cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2010;55:1339–47. doi: 10.1016/j.jacc.2010.01.006. doi:10.1016/j.jacc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. doi:10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szkudelski T, Szkudelska K. Anti-diabetic effects of resveratrol. Ann N Y Acad Sci. 2011;1215:34–9. doi: 10.1111/j.1749-6632.2010.05844.x. doi:10.1111/j.1749-6632.2010.05844.x. [DOI] [PubMed] [Google Scholar]
- 15.Ji G, Wang Y, Deng Y, Li X, Jiang Z. Resveratrol ameliorates hepatic steatosis and inflammation in methionine/choline-deficient diet-induced steatohepatitis through regulating autophagy. Lipids Health Dis. 2015;14:134. doi: 10.1186/s12944-015-0139-6. doi:10.1186/s12944-015-0139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang LR1, Zhu GQ, Shi KQ, Braddock MZM. Autophagy in Ethonol-Exposed Liver Disease. Expert Rev Gastroent Hepatol. 2015;9:1031–103. doi: 10.1586/17474124.2015.1052065. doi:. doi: 10.1586/17474124.2015.1052065. [DOI] [PubMed] [Google Scholar]
- 17.Sambasivarao S V. Overfed Ossabaw swine with early stage metabolic syndrome have normal coronary collateral development in response to chronic ischemia. Basic Res Cardiol. 2012;107:243–64. doi: 10.1007/s00395-012-0243-y. doi:10.1016/j.micinf.2011.07.011.Innate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu LM, Lassaletta AD, Robich MP, Liu Y, Burgess T, Laham RJ, et al. Effects of red wine and vodka on collateral-dependent perfusion and cardiovascular function in hypercholesterolemic swine. Circulation. 2012;126:65–73. doi: 10.1161/CIRCULATIONAHA.111.082172. doi:10.1161/CIRCULATIONAHA.111.082172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potz BA, Sabe AA, Elmadhun NY, Feng J. Calpain inhibition decreases myocardial apoptosis in a swine model of chronic myocardial ischemia. Surgery. 158:445–52. doi: 10.1016/j.surg.2015.03.034. n.d doi:10.1016/j.surg.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unsain N, Barker PA. New Views on the Misconstrued: Executioner Caspases and Their Diverse Non-apoptotic Roles. Neuron. 2015;88:461–74. doi: 10.1016/j.neuron.2015.08.029. doi:10.1016/j.neuron.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, et al. Alcohol stimulates macrophage activation through caspase dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2015 doi: 10.1016/j.jhep.2015.11.020. doi:10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinkel S, Gross A, Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 2006;13:1351–9. doi: 10.1038/sj.cdd.4401987. doi:10.1038/sj.cdd.4401987. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Liu J, Gong Z. Resveratrol induces apoptosis in K562 cells via the regulation of mitochondrial signaling pathways. Int J Clin Exp Med. 2015;8:16926–33. [PMC free article] [PubMed] [Google Scholar]
- 24.Fan S, Zhang B, Luan P, Gu B, Wan Q, Huang X, et al. PI3K / AKT / mTOR / p70S6K Pathway Is Involved in A ? 25-35-Induced Autophagy. Biomed Res Int. 2015:2015. doi: 10.1155/2015/161020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardie DG. The AMP-activated protein kinase pathway - new players upstream and downstream. J Cell Sci. 2004;117:5479–87. doi: 10.1242/jcs.01540. doi:10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 26.Sun L, Zhang S, Yu C, Pan Z, Liu Y, Zhao J, et al. Hydrogen sulfide reduces serum triglyceride by activating liver autophagy via the AMPK-mTOR pathway. Am J Physiol Endocrinol Metab. 2015;309:925–35. doi: 10.1152/ajpendo.00294.2015. doi:10.1152/ajpendo.00294.2015. [DOI] [PubMed] [Google Scholar]
- 27.Tanida I, Ueno T, Kominami E. Human light chain 3/MAP1LC3B Is cleaved at its carboxyl-terminal Met. 121 to expose Gly120 for lipidation and targeting to autophagosomal membranes. J Biol Chem. 2004;279:47704–10. doi: 10.1074/jbc.M407016200. doi:10.1074/jbc.M407016200. [DOI] [PubMed] [Google Scholar]
- 28.Eskelinen E-L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med. 2006;27:495–502. doi: 10.1016/j.mam.2006.08.005. doi:10.1016/j.mam.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Chen M, Zhou Y, Yi L, Gao Y, Ran L, et al. Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. 2015:1443–57. doi: 10.1002/mnfr.201500016. doi:10.1002/mnfr.201500016. [DOI] [PubMed] [Google Scholar]



![Figure 2[A-F]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/957a/4920969/76e4ca272e5e/nihms-772333-f0002.jpg)
![Figure 2[A-F]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/957a/4920969/862244817024/nihms-772333-f0003.jpg)
![Figure 2[A-F]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/957a/4920969/8fc6ad7fe549/nihms-772333-f0004.jpg)
![Figure 2[A-F]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/957a/4920969/8f8cad979a26/nihms-772333-f0005.jpg)
![Figure 4 [A-J]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/957a/4920969/aeb831a9421f/nihms-772333-f0007.jpg)