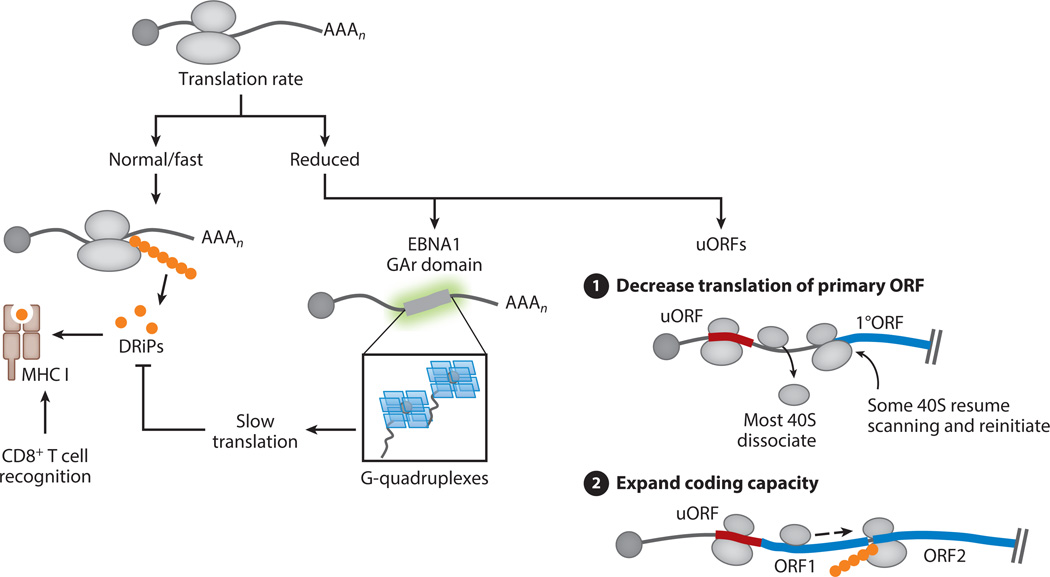
Figure 4.
Controlling the rate of translation as a means to impact antigen processing and regulate viral gene expression. Many antigenic MHC I peptides are generated from rapidly degraded defective ribosomal products (DRiPs) made during translation. Viral proteins may avoid DRiP presentation by reducing their translation rate. The EBNA1 latency protein of Epstein-Barr virus contains a glycine-alanine repeat (GAr) domain that folds into G-quadruplex structures, which decrease EBNA1 translation and correlate with reduced antigen presentation. Viral protein translational efficiency can also be controlled by the presence of short upstream open reading frames (uORFs). (❶) uORFs decrease the translation rate of the primary ORF (1°ORF) by engaging scanning ribosomes, enabling only a subset to reinitiate at the downstream ORF. (❷) uORFs have also been shown to increase viral coding capacity by enabling ribosomes to bypass the 5′ primary ORF and reinitiate at a downstream internal ORF, rendering mRNAs functionally polycistronic. Rapidly degraded peptides produced from uORFs may also be a source of DRiPs.