Abstract
Purpose
Cloacal exstrophy is associated with multiple comorbidities that affect growth. This report describes long-term growth outcomes in a large cohort of patients with cloacal exstrophy and explores associated comorbidities.
Methods
Records of 71 patients with cloacal exstrophy who were treated between 1974 and 2015 were reviewed, and 62 patients with growth data from 2–20 years of age were included. Genetic sex, gender of rearing, and all heights, weights, and comorbidities were noted for each patient. Height-for-age, weight-for-age, and body mass index z-scores (HAZ, WAZ, and BMIZ) were determined using US Centers for Disease Control 2000 growth data, and average patient z-scores were calculated.
Results
There were 904 height and 1301 weight measurements available for 62 patients. 31 were genetically 46,XY, 21 of whom underwent gonadectomy in infancy and were raised female. 46,XX patients, 46,XY male patients, and 46,XY female patients all had median HAZ and WAZ substantially lower than the general population, with median HAZ less than −2, while maintaining normal BMIZ. Short bowel syndrome and enterocystoplasty with intestine were associated with lower z-scores for all parameters.
Conclusions
Patients with cloacal exstrophy have significant multifactorial long-term growth failure. These benchmark data can be used to further optimize management.
Keywords: Cloacal Exstrophy, growth failure, enterocystoplasty
1. Introduction
Cloacal exstrophy is a complex congenital anomaly with an incidence of approximately 1/300,000 live births (1). Although there is considerable variability, cloacal exstrophy classically consists of an omphalocele, imperforate anus, exstrophy of two small hemibladders (between which exists a lateral cecal fissure), and genital malformation. The terminal ileum often prolapses through the exposed cecum, and the hindgut is foreshortened (1, 2). As current survival has improved to nearly 100%, long-term outcomes are of paramount importance (3).
Patients with cloacal exstrophy frequently have comorbidities that affect growth and anecdotally struggle with growth failure. Approximately one quarter of patients with cloacal exstrophy are affected by short bowel syndrome (SBS) of varying severity (4). Almost all patients have some degree of spinal dysraphism, ranging from tethered cord to lipomyelomeningocele. Skeletal abnormalities may affect the spine, pelvis, and lower extremities, with spinal abnormalities (including extra vertebrae, scoliosis, kyphosis) affecting 22–60% of patients, and lower limb anomalies, most commonly congenital talipes equinovarus, seen in 17–26% of patients (4).
Previous studies on the impact of enterocystoplasty on growth outcomes have had mixed findings, with some studies documenting an adverse effect and others demonstrating no effect after adjustment for confounding variables (6). Abnormalities of the upper urinary tract are seen in up to two thirds of patients, and lower urinary tract reconstruction may be complicated by metabolic and infectious sequelae (5). Mild to moderate metabolic acidosis is reported in 15% of patients with incontinent urinary conduits and in approximately half of patients with continent urinary diversions, likely due to the longer segment of bowel used and the longer dwell time of urine within the pouch (5). Gastrocystoplasty does not result in metabolic acidosis, and also has the advantage of enteral sparing for patients who have relatively short bowel (6).
Acidosis in turn impacts growth due to its effect on calcium homeostasis and direct effects on the growth hormone-IGF-1 axis. It results in increased in urine calcium loss (as calcium is committed to H+ as a buffer), increased intestinal sulfate absorption, which inhibits renal tubule calcium reabsorption, and the direct activation of osteoclasts causing growth disturbance and reduced bone mineral density (5, 7). Acidosis also blunts the release of growth hormone, and animal models have shown suppressed serum IGF-1, hepatic IGF-1 mRNA and hepatic growth hormone receptor mRNA, and decreased gene expression of IGF-1 at the growth plate of the long bones (8).
Patients with cloacal exstrophy often have conditions that can impair their ability to physiologically compensate for the metabolic derangements associated with enterocystoplasty. Renal insufficiency is an important risk factor for uncompensated acidosis, and the majority of patients have upper urinary tract anomalies, including renal agenesis, pelvic kidneys, horseshoe kidneys, fusion anomalies, hydronephrosis, or ureteral anomalies (5). A predilection for renal impairment may be compounded by recurrent urinary tract infections (4). In addition, patients may have high GI losses due to congenitally short colon, small intestine, or ostomy, contributing to metabolic derangements. Growth disturbances due to acidosis can be reversed with adequate alkali therapy to correct metabolic acidosis (9).
This report is the first to describe long-term growth outcomes in a large cohort of patients with cloacal exstrophy and explores comorbidities that may be associated with growth failure.
2. Methods
2.1 Study Design
With IRB approval (IRB-P00017268), the Boston Children’s Hospital records of 71 patients with cloacal exstrophy treated at this institution and at Massachusetts General Hospital between 1974 and 2015 were reviewed, and patients who had height and weight recorded between 2 and 20 years of age were included in the study. Of the 62 included patients, 44 patients were followed by a single surgeon (WHH) with extensive expertise in the field. Genetic sex, gender of rearing, and all available height and weight measurements were collected for each patient. The presence and degree of spinal dysraphism, clinical diagnosis of SBS or treatment with home parenteral nutrition (PN), presence and type of lower limb anomalies, type of bladder reconstruction at most recent follow up, use of supplemental growth hormone, and treatment with exogenous estrogens were recorded whenever available. Adult height was recorded for any patient with a height measured at 20 years of age or later, and for patients with multiple adult heights the average of these measurements was used.
2.2 Statistical Methods
Using a publically available program from the United States Centers for Disease Control (CDC) (10), 2000 growth data were used to calculate height-for-age, weight-for-age, and body mass index-for-age z-scores (HAZ, WAZ, and BMIZ) from each measurement. Gestational age was not available for most patients, and therefore height and weight measures prior to two years of age were not included as it was not possible to adjust for prematurity. 46,XY patients who were reared female had both male and female z-scores calculated.
In order to statistically weight patients, the mean z-score was calculated for each patient in each parameter. The Wilcoxon test was used to assess deviation from a normal median of zero for each group, as mean patient z scores were not normally.
HAZ, WAZ, and BMIZ were compared to 15 exposures of interest using generalized estimating equations to account for repeated measurements. These outcomes included presence of any spinal dysraphism, tethered cord, open spinal dysraphism, SBS, home PN use, any lower limb anomaly, documented grossly normal lower extremities, clubfoot, other lower limb anomaly, bladder reconstruction without enterocystoplasty, gastrocystoplasty only (without intestine) reconstruction, any intestine used in bladder reconstruction, stomach used in bladder reconstruction, small bowel used in bladder reconstruction, and large bowel used in bladder reconstruction. Data was analyzed using SPSS, Version 21 (Armonk, New York).
3. Results
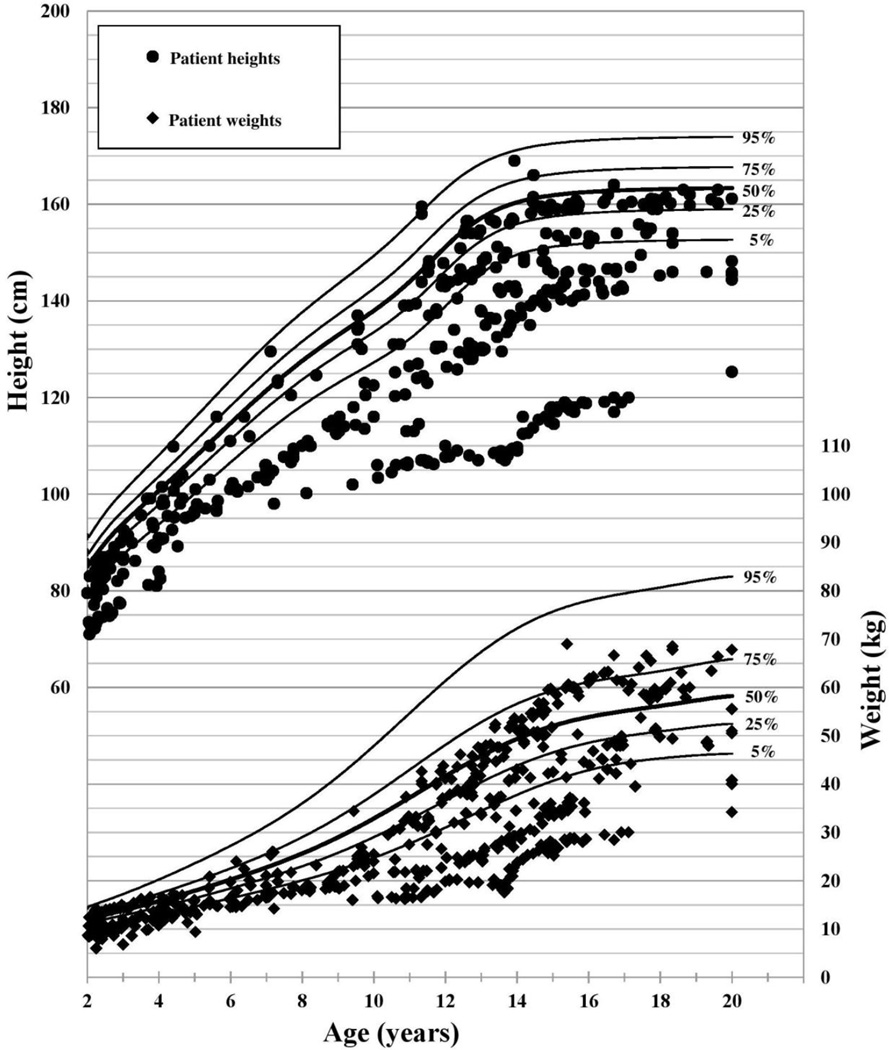
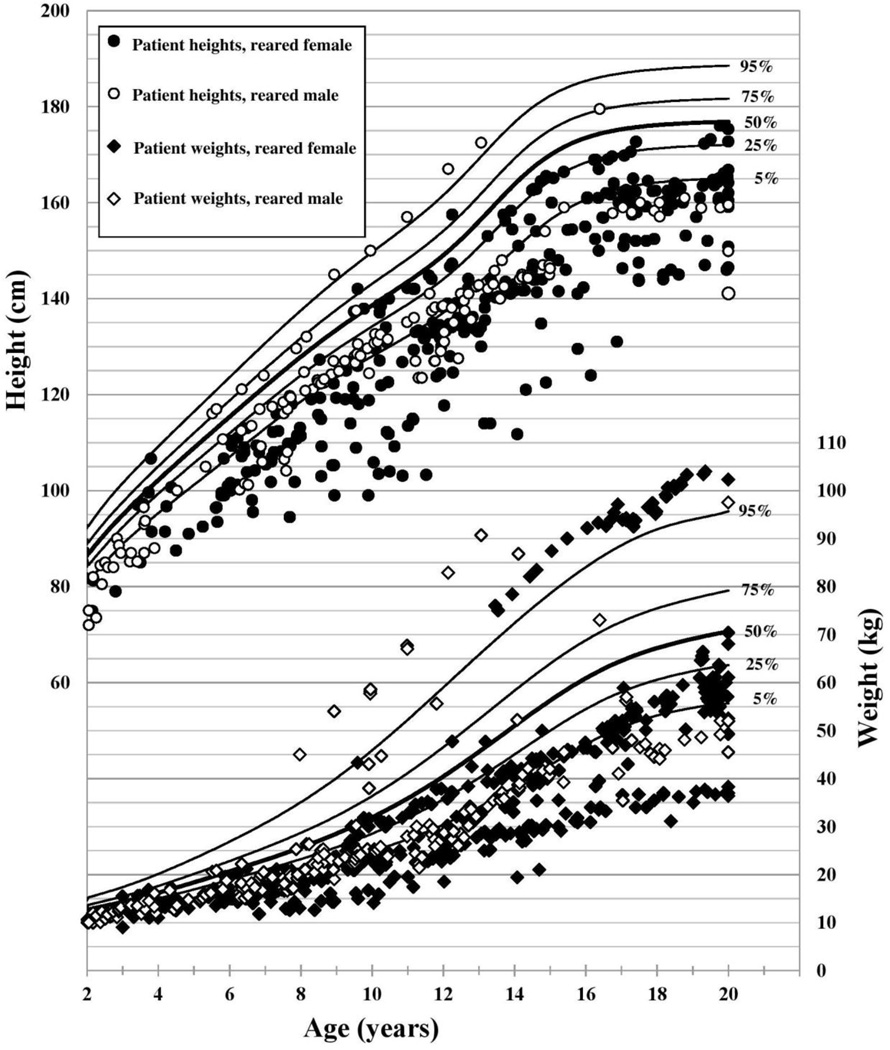
Of the 62 patients, 31 were genetically 46,XX, and 31 patients were genetically 46,XY, 21 of whom underwent gonadectomy in infancy and were raised as females. The other 10 46,XY patients did not undergo gonadectomy and were raised male. Associated conditions are listed in 1. There were 904 height and 1301 weight measurements taken between 2 and 20 years of age (Figures 1 and 2).
Figure 1.
46,XX Patient Stature and Weight with CDC 2000 female growth curves (12)
Figure 2.
46,XY Patient Stature, Weight, and Gender of Rearing with CDC 2000 male growth curves (12)
In all three groups, HAZ was significantly lower than the population norm (Table 1: Growth Outcomes in Patients with Cloacal Exstrophy). At the time of the most recent available height measurement (median age 16.6 years), 46% of patients had short stature as defined by a HAZ of ≤ −2 (11), and 70% had HAZ ≤ −1. Only 16% of the cohort had a HAZ above population median at the time of their most recent height measurement. In the twenty patients with adult height data, average final adult height was 161.9 ±8.8 cm among 46,XY patients reared female, 150.2 ±9.2 cm among 46,XY patients reared male, and 145.1 ±10.5 cm among 46,XX patients. Of note, based on CDC 2000 data, short stature (z-score −2) for adult men is 162.5 cm and for women is 150.3 cm. Average adult height for men is 176.8 cm and for women is 163.3 cm (12).
Table 1.
Growth Outcomes in Patients with Cloacal Exstrophy
| Median | (IQR) | P value | |
|---|---|---|---|
| 46,XX, N=31 | |||
| HAZ | −2.32 | (−3.28 to −1.12) | <0.001* |
| WAZ | −1.24 | (−2.91 to −0.00) | <0.001* |
| BMIZ | −0.16 | (−0.99 to 0.76) | 0.75 |
| 46,XY reared male, N=10 | |||
| HAZ | −2.69 | (−3.63 to −1.34) | 0.021* |
| WAZ | −1.37 | (−1.93 to −0.28) | 0.06 |
| BMIZ | 1 | (−0.99 to 1.69) | 0.31 |
| 46,XY reared female, male growth norms, N=21 | |||
| HAZ | −2.48 | (−3.28 to −1.77) | <0.001* |
| WAZ | −1.72 | (−2.93 to −1.00) | <0.001* |
| BMIZ | −0.38 | (−1.40 to 0.24) | 0.054 |
| 46,XY reared female, female growth norms, N=21 | |||
| HAZ | −2.05 | (−2.95 to −1.08) | <0.001* |
| WAZ | −1.57 | (−2.70 to −0.50) | <0.001* |
| BMIZ | −0.26 | (−1.17 to 0.35) | 0.13 |
Data is reported as median (interquartile range). HAZ = height-for-age z-score, WAZ = weight-for-age z-score, BMIZ = body mass index z-score. All z-scores calculated using Center for Disease Control 2000 stature-for-age, weight-for-age, and BMI-for-age growth curves based on measurements taken ages 2 to 20 years. P values calculated using a 1-sample Wilcoxon test compared to a median value of zero.
Statistically significant.
WAZ was significantly lower in the 46,XX group and the 46,XY reared female group, while the lower WAZ in the 46,XY male group was not significantly different from the population norm (P=0.06). For the 46,XY group that was raised female, significant growth failure was present regardless of whether male or female growth norms were used. BMIZ did not differ significantly from the general population for any group. There were no significant differences in HAZ, WAZ, or BMIZ between the three groups of patients.
All of the 46,XY patients reared female received exogenous estrogen during their teenage years, starting at approximately 14 years of age. In a few patients, the start of estrogen was noted to be delayed until later in the teen years in order to allow time for growth, or in one patient, to pursue psychological evaluation of gender identity. In one of the 46,XY patients reared female an intra-abdominal testis that was not detected during infancy was discovered at 14 years of age after androgenizing effects were noted. This testis was resected prior to initiating exogenous estrogen. Eight patients were treated with supplemental growth hormone injections, for variable periods of time.
Prevalence of the studied comorbidities are included in Table 2. The two comorbidities found to be associated with growth failure were a diagnosis of short bowel syndrome and the type of urinary tract reconstruction. Thirteen patients (21%) had SBS; five of those patients needed periods of home parenteral nutrition. Patients with a documented history of SBS had significantly lower HAZ (p=0.027), lower WAZ (p = 0.033), and lower BMIZ (p=0.05) than patients without documented SBS.
Table 2.
Patient Characteristics, 62 patients with Cloacal Exstrophy
| N | % | |
|---|---|---|
| Karyotype, gender of rearing | ||
| 46,XX, female | 31 | 50.0% |
| 46,XY, female | 21 | 33.9% |
| 46,XY, male | 10 | 16.1% |
| Short bowel syndrome† | ||
| Clinical diagnosis | 13 | 21.0% |
| Home PN use | 5 | 8.1% |
| Spinal dysraphism† | ||
| No spinal dysraphism | 8 | 12.9% |
| Tethered cord | 32 | 51.6% |
| Open spinal dysraphism | 20 | 32.3% |
| Lower extremity exam† | ||
| No gross anomaly | 31 | 50.0% |
| Clubfoot (unilateral or bilateral) | 8 | 12.9% |
| Other lower limb anomaly | 8 | 12.9% |
| Type of lower urinary tract reconstruction | ||
| Bladder only (no enterocystoplasty) | 13 | 21.0% |
| Gastrocystoplasty only (no intestine) | 8 | 12.9% |
| Enterocystoplasty with any intestine | 38 | 61.3% |
| Stomach used | 24 | 38.7% |
| Small intestine used | 33 | 53.2% |
| Large intestine used | 13 | 21.0% |
| Treatment with growth hormone | 8 | 12.9% |
Status of spinal dysraphism was not recorded for 2/62 patients. Type of lower urinary tract reconstruction was not recorded for 3/62 patients. Lower limb exam was not recorded for 15/62 patients, and hip dysplasia was not included as lower limb anomaly.
All patients required extensive urologic surgery. Two previously published articles by Lund and Hendren include illustrations and examples of some of the complex reconstructions used (1, 13). The type of lower urinary tract reconstruction was documented in 95% (59/62) of patients (Table 2). 56 patients had a continent mechanism in place, while two patients had a simple conduit and one had a ureterostomy at most recent follow up. Patients who did not undergo enterocystoplasty, i.e. had native bladder only in the urinary tract reconstruction, had significantly higher HAZ (p=0.041), higher WAZ (p<0.001), and higher BMIZ (p<0.001) when compared with patients who had enterocystoplasty with any combination of bowel or stomach. The use of small bowel, large bowel, or both was associated with a lower WAZ (p=0.006) and lower BMIZ (p=0.018) but no difference in HAZ (p=0.10) when compared with patients who had bladder-only or gastrocystoplasty-only reconstructions. Patients with any gastrocystoplasty, with or without bowel, had a lower HAZ (p=0.004), but did not differ from the rest of the group with respect to WAZ (p=0.070) or BMIZ (p=0.64).
Genetic sex, gender of rearing, presence or degree of spinal dysraphism, and presence or type of lower limb anomaly showed no statistically significant association with z-scores.
4. Discussion
In a large cohort of patients with cloacal exstrophy followed over 42 years, we found a high prevalence of growth failure, with nearly half of patients having short stature with height-for-age z-score < −2, a finding which was consistent regardless of sex or gender of rearing. The growth failure observed in this cohort is likely multifactorial. We identified short bowel syndrome and type of urinary tract reconstruction as important risk factors for growth outcomes.
Historically, gonadectomy in infancy with gender reassignment to female was standard of care for 46,XY patients born with cloacal exstrophy due to the difficulty in constructing a phallus for these patients, who are generally born with widely spaced hemiphalluses and minimal native penile tissue (14). These patients were then reared in the female gender, and were given exogenous estrogens as adolescents in order to induce a female puberty, a therapy which has important implications for growth. Due to research which has raised concerns about androgen imprinting in the fetal and infant period, this treatment pattern has shifted such that most practitioners now do not routinely recommend gonadectomy, and 46,XY patients are more often raised male (15).
A surprising finding of this study is that 46,XY patients raised female had the highest mean adult height of all groups. Although the limited sample size precluded robust statistical comparison of adult height between groups, and mean childhood height z-scores were comparable, this finding nevertheless prompted us to consider potential reasons for this difference. For 46,XY patients raised female, the timing of pubertal induction with exogenous hormones and the presence of the Y chromosome may impact growth patterns (16). Estrogen induces stimulation of the growth hormone- insulin-like growth factor-1(GH-IGF-I1) axis and directly interacts with the growth plate (17). The initiation of exogenous estrogen at age 14 for 46,XY female patients is much later than the age of female puberty in the general population; typically, a diagnosis of delayed puberty in girls is made if there is no breast development by 13 years of age, which is 2–2.5 standard deviations later than the population mean (18). This delay in the administration of exogenous estrogens may increase adult height by allowing for more growth before epiphyseal closure (19).
SBS may affect growth due to nutritional stunting or micronutrient malabsorption, and all patients with cloacal exstrophy typically have a severely shortened hindgut. Critical portions of the small intestine, especially the terminal ileum, may be congenitally absent, surgically resected, or repurposed to reconstruct the bladder or vagina. Micronutrient deficiencies including in copper, zinc, phosphorus, calcium, vitamin D, or manganese can adversely affect growth (20). In addition to nutritional deficiencies, children who lack a functional colon have excess sodium losses in their stool and may develop a total-body sodium deficit, resulting in growth retardation (21, 22). This phenomenon is documented both for patients who have ostomies and those with ileoanal anastomoses, and may exist in the context of normal serum electrolytes (23). Total body sodium status is best assessed by urine sodium, which should be routinely assessed in at-risk children with poor growth (22, 24).
In this study insufficient data were available to determine the chronic acid-base status of the patients, thus precluding the ability to directly assess the effect of metabolic acidosis on growth in this cohort. It is therefore difficult to determine whether the different growth outcomes associated with different techniques of urinary reconstruction in this cohort may be partially attributable to the metabolic and nutritional consequences of enterocystoplasty, vs. the underlying phenotype of the patients that lead to their individualized reconstructions.
Patients with cloacal exstrophy have numerous comorbidities that may negatively impact growth outcomes, and the relative contributions of these comorbities are not clearly defined. The retrospective nature of this study limits the ability to accurately assess for the presence of comorbidities, as not all patients had complete data available. Therefore, the prevalence of some associated conditions may be underreported, obscuring any relationship with outcomes. Different growth outcomes observed in patients with SBS or the various urinary tract reconstruction techniques may be due to the patient’s underlying phenotype, with these variables as a marker for severity, rather than a direct cause of the growth disparity. More severely affected patients may be overrepresented as they would be more likely to present for care, and weight measurements in particular may be falsely low if measured during acute illnesses. Spinal dysraphism, scoliosis, kyphosis, or other vertebral anomalies may affect height. The presence of lower limb anomalies may also affect the ability to record accurate heights. In these patients, arm span can be a useful growth parameter to track, though stature may remain an important outcome for the patient.
5. Conclusion
Patients with cloacal exstrophy have significant long-term growth failure, which is likely multifactorial. The benchmark data from this study can be used as a basis to optimize the multidisciplinary management of these complex patients. Specific nutritional recommendations include optimizing support (especially at times of physiologic stress) and monitoring and, if necessary, replacing micronutrients and total body sodium. Metabolic consequences of enterocystoplasty should be considered at the time of surgery and closely monitored thereafter, with alkali therapy to correct acidosis when applicable. The effect of exogenous hormones on growth potential should be taken into account when determining timing and dosage of exogenous hormones for agonadal patients.
Acknowledgments
CD was supported in part by K24 DK104676-06
Footnotes
Role of the Authors:
Fullerton: Conception/design, data acquisition, analysis and interpretation, participated in drafting, gave final approval
Sparks: Conception/design, data acquisition, analysis and interpretation, participated in revision, gave final approval
Hall: analysis and interpretation, participated in revision, gave final approval
Chan: analysis and interpretation, participated in revision, gave final approval
Duggan: Conception/design, analysis and interpretation, participated in revision, gave final approval
Lund: Data acquisition, analysis and interpretation, participated in revision, gave final approval
Modi: Conception/design, analysis and interpretation, participated in revision, gave final approval
Jaksic: Conception/design, analysis and interpretation, participated in revision, gave final approval
Hendren: Conception/design, data acquisition, analysis and interpretation, participated in revision, gave final approval
References
- 1.Lund DP, Hendren WH. Cloacal exstrophy: a 25-year experience with 50 cases. Journal of pediatric surgery. 2001;36(1):68–75. doi: 10.1053/jpsu.2001.20009. [DOI] [PubMed] [Google Scholar]
- 2.Sawaya D, Goldstein S, Seetharamaiah R, Suson K, Nabaweesi R, Colombani P, et al. Gastrointestinal ramifications of the cloacal exstrophy complex: a 44-year experience. Journal of pediatric surgery. 2010;45(1):171–175. doi: 10.1016/j.jpedsurg.2009.10.030. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 3.Phillips TM. Spectrum of cloacal exstrophy. Seminars in pediatric surgery. 2011;20(2):113–118. doi: 10.1053/j.sempedsurg.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Woo LL, Thomas JC, Brock JW. Cloacal exstrophy: a comprehensive review of an uncommon problem. Journal of pediatric urology. 2010;6(2):102–111. doi: 10.1016/j.jpurol.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Stein R, Schroder A, Thuroff JW. Bladder augmentation and urinary diversion in patients with neurogenic bladder: non-surgical considerations. Journal of pediatric urology. 2012;8(2):145–152. doi: 10.1016/j.jpurol.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert SM, Hensle TW. Metabolic consequences and long-term complications of enterocystoplasty in children: a review. The Journal of urology. 2005;173(4):1080–1086. doi: 10.1097/01.ju.0000155248.57049.4e. [DOI] [PubMed] [Google Scholar]
- 7.Stein R, Rubenwolf P. Metabolic consequences after urinary diversion. Frontiers in pediatrics. 2014;2:15. doi: 10.3389/fped.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth KS, Chan JC. Renal tubular acidosis: a new look at an old problem. Clinical pediatrics. 2001;40(10):533–543. doi: 10.1177/000992280104001001. [DOI] [PubMed] [Google Scholar]
- 9.Chang CY, Lin CY. Failure to thrive in children with primary distal type renal tubular acidosis. Acta paediatrica Taiwanica = Taiwan er ke yi xue hui za zhi. 2002;43(6):334–339. [PubMed] [Google Scholar]
- 10.A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years) Atlanta, GA: U.S. Department of Health & Human Services; 2015. [Google Scholar]
- 11.Sperling M. Pediatric endocrinology. 4th. Philadelphia, PA: Saunders/Elsevier; 2014. p. xv.p. 889. [Google Scholar]
- 12.CDC Growth Charts. Atlanta GA: Centers for Disease Control and Prevention, National Center for Health Statistics; 2010. [cited 2015]. http://www.cdc.gov/growthcharts/cdc_charts.htm. [Google Scholar]
- 13.Lund DP, Hendren WH. Cloacal exstrophy: experience with 20 cases. Journal of pediatric surgery. 1993;28(10):1360–1368. doi: 10.1016/s0022-3468(05)80328-8. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 14.Husmann DA, McLorie GA, Churchill BM. Phallic reconstruction in cloacal exstrophy. The Journal of urology. 1989;142(2 Pt 2):563–564. doi: 10.1016/s0022-5347(17)38816-x. discussion 72. [DOI] [PubMed] [Google Scholar]
- 15.Diamond DA, Burns JP, Huang L, Rosoklija I, Retik AB. Gender assignment for newborns with 46XY cloacal exstrophy: a 6-year followup survey of pediatric urologists. The Journal of urology. 2011;186(4 Suppl):1642–1648. doi: 10.1016/j.juro.2011.03.101. [DOI] [PubMed] [Google Scholar]
- 16.Tosson H, Rose SR, Gartner LA. Children with 45,X/46,XY karyotype from birth to adult height. Hormone research in paediatrics. 2010;74(3):190–200. doi: 10.1159/000281468. [DOI] [PubMed] [Google Scholar]
- 17.Shim KS. Pubertal growth and epiphyseal fusion. Ann Pediatr Endocrinol Metab. 2015;20(1):8–12. doi: 10.6065/apem.2015.20.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmert MR, Dunkel L. Clinical practice. Delayed puberty. The New England journal of medicine. 2012;366(5):443–453. doi: 10.1056/NEJMcp1109290. [DOI] [PubMed] [Google Scholar]
- 19.Wit JM, Balen HV, Kamp GA, Oostdijk W. Benefit of postponing normal puberty for improving final height. Eur J Endocrinol. 2004;151(Suppl 1):S41–S45. doi: 10.1530/eje.0.151s041. [DOI] [PubMed] [Google Scholar]
- 20.Sonneville K, Duggan C. Manual of pediatric nutrition. Fifth. Shelton, Connecticut: People's Medical Publishing House-USA; 2014. p. xi.p. 635. [Google Scholar]
- 21.Kennedy HJ, Al-Dujaili EA, Edwards CR, Truelove SC. Water and electrolyte balance in subjects with a permanent ileostomy. Gut. 1983;24(8):702–705. doi: 10.1136/gut.24.8.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butterworth SA, Lalari V, Dheensaw K. Evaluation of sodium deficit in infants undergoing intestinal surgery. Journal of pediatric surgery. 2014;49(5):736–740. doi: 10.1016/j.jpedsurg.2014.02.057. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz KB, Keating JP, Ternberg JL, Bell MJ, Howald MA. Sodium balance following Soave ileo-endorectoal pull-through. Journal of pediatric surgery. 1977;12(6):945–953. doi: 10.1016/0022-3468(77)90605-4. [DOI] [PubMed] [Google Scholar]
- 24.O'Neil M, Teitelbaum DH, Harris MB. Total body sodium depletion and poor weight gain in children and young adults with an ileostomy: a case series. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2014;29(3):397–401. doi: 10.1177/0884533614528543. [DOI] [PubMed] [Google Scholar]