Abstract
Objective. Prolonged exposure to opioids is known to produce neuroplastic changes in animals; however, few studies have investigated the effects of short-term prescription opioid use in humans. A previous study from our laboratory demonstrated a dosage-correlated volumetric decrease in the right amygdala of participants administered oral morphine daily for 1 month. The purpose of this current study was to replicate and extend the initial findings.
Methods. Twenty-one participants with chronic low back pain were enrolled in this double-blind, placebo-controlled study. Participants were randomized to receive daily morphine (n = 11) or a matched placebo (n = 10) for 1 month. High-resolution anatomical images were acquired immediately before and after the treatment administration period. Morphological gray matter changes were investigated using tensor-based morphometry, and significant regions were subsequently tested for correlation with morphine dosage.
Results. Decreased gray matter volume was observed in several reward- and pain-related regions in the morphine group, including the bilateral amygdala, left inferior orbitofrontal cortex, and bilateral pre-supplementary motor areas. Morphine administration was also associated with significant gray matter increases in cingulate regions, including the mid cingulate, dorsal anterior cingulate, and ventral posterior cingulate.
Conclusions. Many of the volumetric increases and decreases overlapped spatially with the previously reported changes. Individuals taking placebo for 1 month showed neither gray matter increases nor decreases. The results corroborate previous reports that rapid alterations occur in reward-related networks following short-term prescription opioid use.
Keywords: Opioids, Magnetic Resonance Imaging, Tensor-Based Morphometry
Introduction
The use of prescription opioid medications has increased significantly in recent years. Driven by a greater reliance on potent analgesics for the treatment of chronic pain, consumption in the United States rose 300% between 1999 and 2010 [1]. Opioid medications are among the most commonly dispensed class of drugs, second only to cardiovascular and antihyperlipidemic medications [1]. The risks associated with opioid use include analgesic tolerance, cognitive impairment, sleep disturbance, hyperalgesia, and addiction [2–5]. These potential side effects suggest that opioids have an impact on the central nervous system. Furthermore, chronic opioid exposure has been shown to induce neuroplastic changes in animals [6–8]. However, the effects of short-term opioid use at analgesic doses in humans are not well understood.
Current human data are largely gathered from opioid-dependent participants who are assessed cross-sectionally, rather than longitudinally. One study of prescription opioid-dependent participants reported decreased gray matter volume in the bilateral amygdala [9], a key reward-modulating area involved in impulse control, addiction, and tolerance [10–12]. It is unknown, however, what degree of exposure to opioids is required to drive such a neuroplastic change. As opioid use rises, so does the need for a better understanding of the neuropsychological and neuroanatomical effects of these drugs during compliant medical use.
The first longitudinal study of prescription opioid effects on the brain from our laboratory [13] demonstrated a dosage-correlated volumetric decrease in the right amygdala of participants administered oral morphine daily for 1 month. Furthermore, dosage-correlated volumetric increases were observed in the right hypothalamus, left inferior frontal gyrus, right ventral posterior cingulate, and right caudal pons. The primary aim of this magnetic resonance imaging (MRI) study was to replicate and extend the initial findings, with the inclusion of a randomized placebo group to control for the effects of expectancy on brain structure. Individuals with chronic low back pain underwent structural scans of the brain both before and after 1 month of daily morphine or placebo therapy in order to investigate any morphine-associated morphological changes in gray matter.
Methods
Participants
Twenty-one participants (14 male, 7 female; mean age 41.9 years, SD = 10) with chronic, moderate-to-severe, nonradicular, low back pain (mean duration with pain 8.1 years, SD = 7.7) were invited to participate in this study. Inclusion criteria were the same as used in a previous study [4]. Participants were excluded on the basis of: 1) a history of substance abuse, including cannabis or nicotine; 2) previous use of opioids; 3) a current or lifetime diagnosis of Axis I psychiatric disorders; and 4) any evidence of neuropathic pain or a prescription for neuropathic pain medication. However, participants were permitted to continue taking over-the-counter analgesics for the duration of the study. All study procedures were approved by the Institutional Review Board at Stanford University School of Medicine, and written informed consent was obtained from all participants before this research was undertaken.
Medication
The overall design of this double-blind, placebo-controlled study was as follows: participants first attended a baseline (pre-morphine) scanning session. Participants were randomly assigned to receive placebo or morphine therapy. Morphine was titrated as per a previously described procedure [4]. A double-encapsulated, sustained-release oral formulation of morphine was used (MS-Contin; Purdue Frederick, Stamford, CT, USA). The initial dose for all participants in the morphine group was 15 mg, twice daily. Every 2 days, the daily dosage was increased by 15 mg. Titration was discontinued when: 1) adequate analgesia had been achieved; 2) side effects limited further dosage increases; or 3) an upper maximum dosage of 120 mg/day (8 capsules) was reached. For ethical and medical safety reasons, doses of morphine were not randomly assigned. Medication was administered for 1 month; total morphine exposure over the month for the morphine group (n = 11) ranged from 960 to 2,865 mg (mean 2,152 mg).
A non-active placebo was chosen for this study as the structural effects of potential active placebo agents are unknown. Participants in the placebo group (n = 10) received matched, double-encapsulated placebo capsules for the duration of the study. The ‘dose’ was titrated according to the same criteria as the morphine group, up to a maximum of eight capsules per day. After 1 month, all participants returned for a second scan session.
Pain Measure
In order to assess the effect of morphine on chronic pain, the Brief Pain Inventory [14] was administered at each scan. The pain intensity subscale of the Brief Pain Inventory was used as a measure of pain severity. Pain intensity score was calculated by taking a mean of four items (average, least, and worst pain over the last 24 hours, as well as present pain) scored on a 10-point numerical rating scale. The developers of the Brief Pain Inventory recommend a composite of the four pain items to represent pain severity as the models for validation were based on all four items [15].
Image Acquisition
Data were collected at the Richard M. Lucas Center for Imaging at Stanford University, using a 3.0 T GE Healthcare Discovery 750 (GE Signa, Milwaukee, WI, USA) and an eight-channel head coil. A T1-weighted 3D inversion recovery-prepared fast spoiled gradient-recalled (IR-FSPGR) scan was acquired (axial slices, repetition time = 7.2 ms, echo time = minimum, flip angle = 11°, 128 slices, slice thickness = 1.2 mm, field of view = 220 × 220 mm, matrix = 256 × 256), yielding 1.2 × 0.86 × 0.86 mm voxel resolution.
Image Analysis
All data were analyzed using a tensor-based morphometry (TBM) technique that tracks local tissue changes in the human brain by applying a non-linear deformation field to align serial MRI scans [16]. Rates of brain change can then be inferred from the local analysis of the applied expansion or compression. Analysis was performed in SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK) within MATLAB (MathWorks, Natick, MA, USA). Pairs of high-resolution images (pre- and post-morphine) from each participant were rigidly coregistered and then segmented into gray and white matter. An average high-resolution image was also calculated, segmented, and manually edited to remove any remaining non-brain tissue. The resultant average gray matter image was then used to mask the pre- and post-morphine gray matter images. The image pairs were then matched precisely using high-dimensional deformation fields [17], yielding a Jacobian determinant (difference) map for each pre-post pair. The edited average gray matter images from each participant were processed with Diffeomorphic Anatomical Registration using Exponentiated Lie Algebra (DARTEL) [18] to generate a spatially normalized group template. The resultant participant-specific warps were applied to the Jacobian determinant maps, resampled into 1 mm isotropic voxel size, smoothed with a 4 mm full width at half maximum Gaussian isotropic kernel, and registered into common Montreal Neurological Institute (MNI) stereotactic space.
Statistical Analysis
To identify regions of opioid-induced gray matter volumetric change, whole brain analyses were performed using a nonparametric, permutation toolbox for SPM, known as Statistical NonParametric Mapping (SnPM13)[19]. The main effect of time was calculated for each group using a multiple-subject single-condition design. Tests were performed using a pseudo T-statistic incorporating variance smoothing with a Gaussian kernel of width 4 mm, and a significance threshold of P < 0.0005 (uncorrected) that corresponded to a false discovery rate of 0.01. A separate cluster threshold of 150 voxels was also used to further reduce false positives.
To determine the relationship between morphological change, morphine exposure, and pain measures, clusters demonstrating significant gray matter changes were tested (two-tailed) for associations with morphine dosage (mg/kg) and pain measures (change in mean pain intensity scores) using Spearman’s rho (rs). An additional false discovery rate of 0.05 was used to correct for the number of correlational tests conducted (equivalent to uncorrected P < 0.0108).
Interaction analyses were performed to investigate whether morphological changes observed in the morphine group differed from the placebo group; however, due to the sample size, we did not have sufficient power to perform whole brain analyses. Therefore, these analyses were restricted to clusters that showed a significant main effect of time in the morphine group. Time * group interactions were tested using a Mann-Whitney test, and an additional false discovery rate of 0.05 was used to correct for the number of tests conducted (equivalent to uncorrected P < 0.0127).
Results
Demographics
Overall, the groups did not differ significantly in age (morphine: 39 ± 10.0 years; placebo: 45 ± 9.5 years; P = 0.178) or duration of pain (morphine: 11.1 ± 9.2 years; placebo: 5.2 ± 5.0 years; P = 0.115). None of the participants had undergone surgery.
Pain Measure
Mean pain intensity was 3.8 ± 1.0 for the morphine group and 4.7 ± 0.8 for the placebo group, indicating mild-to-moderate pain for the morphine group and moderate pain for the placebo group. There was no statistically significant main effect for treatment group (F(1,19) = 3.189, P = 0.090). There was a significant main effect of time on mean pain intensity scores (F(1,19) = 11.813, P = 0.003), with overall pain decreasing during the 1-month study period (morphine pain reduction: 1.52 ± 2.40; placebo pain reduction: 1.46 ± 1.39). These corresponded to a 29.9% and 33.3% reduction in pain intensity in the morphine and placebo groups, respectively. The time * group interaction was not significant (F(1,19) = 0.005, P = 0.945), showing that the rate of pain reduction was similar between those taking morphine and those taking placebo.
Imaging Results
Over the 1-month study period, participants who received morphine treatment exhibited significant volumetric loss and gain in several brain regions (Table 1). Decreased gray matter was observed in the left inferior orbitofrontal cortex, right gyrus rectus, the bilateral pre-supplementary motor areas, and left dorsal posterior cingulate. Volumetric changes in the left dorsal posterior cingulate were significantly correlated with morphine consumption, where higher doses of morphine were associated with larger volumetric decreases in this region.
Table 1.
Regions demonstrating significant gray matter increase or decrease after 1 month of daily morphine administration
| Region | Main effects (morphine group) |
Interaction |
||||
|---|---|---|---|---|---|---|
| MNI coordinates | T-value | rs (P) BPI | rs (P) dosage | Mann-Whitney U | P value | |
| Volume decrease | ||||||
| L dorsal posterior cingulate | −5, −20, 35 | −9.15 | −0.662 (0.026) | −0.782 (0.004)* | 42 | 0.387 |
| L superior temporal gyrus | −44, −23, 5 | −7.22 | −0.078 (0.821) | 0.173 (0.612) | 36 | 0.197 |
| −55, 7, −11 | −6.51 | −0.379 (0.250) | −0.027 (0.937) | 41 | 0.349 | |
| R amygdala | 15, −6, −23 | −7.09 | −0.210 (0.535) | −0.145 (0.670) | 17 | 0.006** |
| L insula | −37, 9, −6 | −7.02 | −0.288 (0.391) | −0.664 (0.026) | 53 | 0.918 |
| −38, 0, −1 | −5.59 | −0.178 (0.600) | −0.209 (0.537) | 42 | 0.387 | |
| L amygdala | −14, −7, −22 | −6.70 | 0.009 (0.979) | −0.455 (0.160) | 62 | 0.654 |
| L pre-supplementary motor area | −8, 9, 69 | −6.09 | −0.023 (0.947) | −0.225 (0.450) | 47 | 0.605 |
| R precentral gyrus | 56, 5, 27 | −6.06 | −0.018 (0.957) | −0.482 (0.133) | 44 | 0.468 |
| R pre-supplementary motor area | 10, 4, 71 | −5.98 | −0.178 (0.600) | −0.355 (0.285) | 58 | 0.863 |
| R gyrus rectus (BA 11) | 5, 62, −19 | −5.64 | −0.667 (0.025) | −0.582 (0.060) | 53 | 0.918 |
| R superior frontal gyrus | 25, 46, 35 | −5.64 | −0.091 (0.789) | −0.318 (0.340) | 12 | 0.006** |
| L inferior orbitofrontal cortex | −35, 21, −14 | −5.50 | 0.274 (0.415) | 0.055 (0.873) | 31 | 0.099 |
| R inferior temporal gyrus | 52, 1, −34 | −5.28 | −0.114 (0.738) | −0.018 (0.958) | 32 | 0.114 |
| R rolandic operculum | 55, −2, 10 | −4.54 | 0.087 (0.800) | 0.036 (0.915) | 29 | 0.072 |
| Volume increase | ||||||
| L insula | −41, 19, −4 | 8.85 | 0.365 (0.269) | 0.136 (0.689) | 62 | 0.654 |
| −42, −15, 2 | 8.66 | 0.078 (0.821) | 0.209 (0.537) | 88 | 0.020 | |
| L anterior cingulate | 1, 35, 26 | 8.41 | 0.155 (0.649) | 0.664 (0.026) | 60 | 0.756 |
| R dorsal anterior cingulate (BA 32) | 1, 43, 0 | 8.20 | −0.137 (0.688) | 0.164 (0.631) | 71 | 0.282 |
| L precuneus | 0, −48, 53 | 7.39 | 0.018 (0.957) | 0.391 (0.235) | 50 | 0.756 |
| R insula | 47, −5, 1 | 7.11 | 0.174 (0.610) | 0.537 (0.066) | 77 | 0.132 |
| R postcentral gyrus | 55, −23, 52 | 6.96 | 0.068 (0.841) | 0.182 (0.593) | 79 | 0.099 |
| R hippocampus | 13, −5, −16 | 5.82 | −0.023 (0.947) | 0.155 (0.650) | 64 | 0.557 |
| R superior temporal gyrus | 64, −17, 12 | 5.40 | −0.575 (0.064) | −0.173 (0.612) | 33 | 0.132 |
| R temporal pole | 27, 9, −25 | 5.20 | 0.521 (0.101) | 0.591 (0.056) | 56 | 1.000 |
| L cerebellum lobule IV | −16, −50, −19 | 5.02 | −0.274 (0.415) | 0.100 (0.770) | 88 | 0.020 |
| L ventral posterior cingulate | −3, −31, 48 | 4.70 | −0.128 (0.708) | 0.564 (0.071) | 56 | 1.000 |
| R fusiform gyrus | 21, −59, −13 | 4.55 | −0.479 (0.136) | −0.173 (0.612) | 63 | 0.605 |
MNI = Montreal Neurological Institute; BPI = Brief Pain Inventory; BA = Brodmann Area; R = right; L = left.
Changes in all regions survived a height-level threshold corrected with a false discovery rate of 0.01 (uncorrected P < 0.0005), and a cluster threshold of 150 contiguous voxels.
Regions showing a main effect of time in the morphine group are listed first, followed by MNI coordinates of the peak voxel, t-value of the peak voxel, and correlation with pain intensity scores and morphine dosage.
All regions that showed a main effect in the morphine group were tested for interaction effects with the placebo group. Mann-Whitney U values are displayed, followed by the P value.
*Denotes significance with a false discovery rate of 0.05 (uncorrected P < 0.0108)
**Denotes significance with a false discovery rate of 0.05 (uncorrected P < 0.0127).
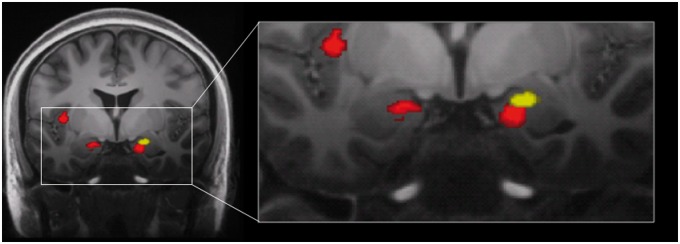
Significant volumetric loss was also seen in the superficial subregions of the bilateral amygdala, approximately in the ventral and posterior cortical nuclei [20]. The location of the cluster in the right amygdala overlapped spatially with the amygdalar volume loss reported previously by Younger et al. [13], but was located inferior and medial to the earlier reported cluster [21] (Figure 1).
Figure 1.
Coronal view (y = -6) of gray matter volume decreases following 1 month of daily morphine. Images of morphine-associated volumetric decreases from the current study (red) and the previous study (yellow) by Younger et al. [13] are overlaid on a 7 T structural image, depicting spatial locations of amygdalar changes. Images are thresholded at voxel-level false discovery rate of P < 0.01.
Additional regions that demonstrated significant volumetric decreases included the left insula, two regions within the left superior temporal gyrus, right precentral gyrus, right superior frontal gyrus, right inferior temporal gyrus, and right rolandic operculum. One cluster within the superior temporal gyrus was located adjacent to the Sylvian fissure, just superior to Brodmann Area 22 while the other was more inferior and lateral, adjacent to the superior temporal sulcus.
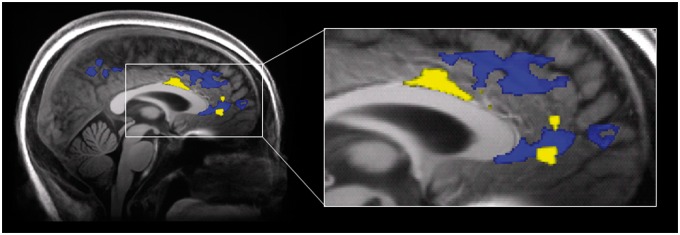
Morphine administration was associated with significant increases in gray matter in a number of regions that showed overlap with previously reported findings. These included regions within the mid cingulate, dorsal anterior cingulate (Figure 2), and ventral posterior cingulate. Gray matter increases were also observed in the bilateral insula, left precuneus, right postcentral gyrus, right hippocampus, right superior temporal gyrus, right temporal pole, and right fusiform gyrus. One cluster within the left insula encompassed the anterior portion, immediately superior to the Sylvian fissure and extended laterally to the left temporal pole and anteriorly the inferior frontal gyrus. The second cluster within the left insula was located within the posterior portion, extending laterally to the left superior temporal gyrus.
Figure 2.
Sagittal view (x = 2) of gray matter volume increases following 1 month of daily morphine. Images of morphine-associated volumetric increases from the current study (blue) and the previous study (yellow) by Younger et al. [13] are overlaid on a 7 T structural image, depicting spatial locations of changes in the pregenual cingulate. Images are thresholded at voxel-level false discovery rate of P < 0.01.
The placebo group showed no significant volumetric increases over time. Likewise, the placebo group showed no volumetric decreases over time.
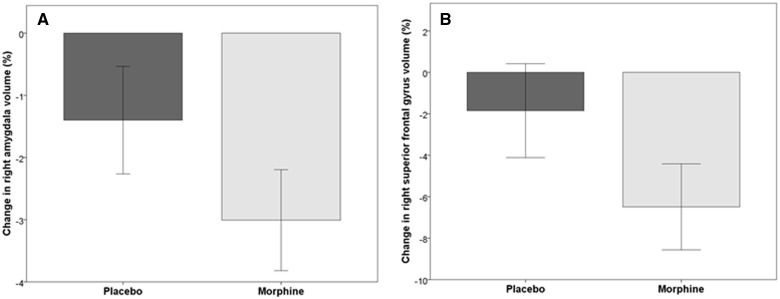
As a post-hoc test, clusters identified in the morphine group were tested against the placebo group in a time * group interaction analysis (Table 1). After a false discovery rate correction (P < 0.05), the following regions showed significant separation of volume change between groups: the right amygdala and the right superior frontal gyrus (Figure 3).
Figure 3.
Bar graphs showing percent volumetric change from baseline in the (A) right amygdala and (B) right superior frontal gyrus for the placebo group (left bar) and morphine group (right bar). Error bars represent 95% confidence intervals. At a voxel-level false discovery rate of P < 0.05, gray matter volume is significantly decreased in the right amygdala and right superior frontal gyrus after morphine exposure and the placebo group shows no significant change.
Discussion
The primary aim of this study was to investigate morphine-associated morphological changes in gray matter after 1 month of daily morphine therapy and, with the inclusion of a randomized placebo group, replicate and extend our previously reported findings in an independent sample. We also aimed to determine whether these morphological changes were associated with morphine exposure and/or pain relief.
We found that 1 month of daily morphine administration was associated with volumetric gray matter changes in several brain regions, many of which had been previously reported by our group. We found decreased gray matter volume in the bilateral amygdala, with the area of the right amygdala overlapping spatially with previously reported amygdalar volume loss. As seen in Figure 1, however, the clusters identified between the two studies are not identical, with the most recent results showing the cluster to be more inferior and medial. The degree of gray matter loss was approximately 3% in both studies: 2.78% (95% CI 1.73–3.03%) for the first study and 3.00% (95% CI 2.20–3.82%) for the present study. The right amygdala was one of only two regions to be significantly different from the placebo group in a post-hoc interaction analysis controlled for multiple comparisons. Gray matter decreases were found in other reward-processing regions such as the inferior and ventromedial orbitofrontal cortex (gyrus rectus) and insula. The orbitofrontal cortex, which shares reciprocal connections with the amygdala, is related to learning and memory of the reward value of reinforcers [22] and has been shown to be activated by nonmonetary reward in opiate addicts [23]. Lyoo et al. [24] observed decreased gray matter density in bilateral insula of opiate-dependent participants, and Daglish et al. [25] reported a positive association between orbitofrontal and insular activation during cue-induced opiate craving. In a more recent study, decreased functional connectivity between anterior insular and amygdala regions was found to be important in distinguishing opioid-dependent individuals from healthy controls [9]. The morphological changes observed in these regions lend support to the preliminary evidence from our first study for rapid alterations in reward-related networks following short-term opioid exposure.
Decreases in gray matter were also observed in a number of structures within pain-processing networks, including the posterior cingulate, superior frontal gyrus, and supplementary motor area. These regions are involved in various aspects of motor control, stimulus localization, and intensity processing [26–28]; however, volumetric changes in these areas did not correlate with reductions in pain scores. All participants in the morphine group showed gray matter decreases in the left dorsal posterior cingulate that were negatively correlated with morphine dosage. However, given the sample size, further independent studies are needed to validate these findings.
Similarly to our previous study, gray matter increases were widely distributed through the brain and generally located outside reward-processing networks. Increased gray matter was observed in a number of the same regions within the cingulate cortex—middle, dorsal anterior, and ventral posterior—that are known to have high mu-opioid receptor density and high mu-opioid binding [21,29].
One important question that arose from our previous study was whether the observed morphological changes were directly related to the resolution of participants’ low back pain. In this study, no significant gray matter volume changes were associated with placebo treatment, despite participants in this group reporting a significant reduction in pain intensity. This observation coupled with the finding that decreased gray matter volume in the dorsal posterior cingulate was significantly correlated with morphine dosage (but not pain intensity) suggests that the brain changes we observed in this study were more likely to be a result of the engagement of reward circuitry rather than from pain reduction alone. However, there exists some similarity and overlap of the underlying neurobiological mechanisms of pain and reward [30,31]. Therefore, changes in brain volume may not necessarily be harmful or adverse, and could reflect clinically relevant effects of opioid treatment.
A number of limitations should be considered in the interpretation of this study. First, the sample size is small; therefore, we cannot make conclusions about the generalizability of these results. Statistical power is low, especially for interaction analyses between groups, and post-hoc correlational analyses. We employed false discovery rate corrections on two levels, as well as a cluster size threshold. As a result, our confidence in statistically significant results is high, though there are likely false negatives. Second, while the participants were matched for pain condition and severity, they were not matched for age and gender. Participants were also permitted to continue taking over-the-counter analgesics during the study that were not matched or controlled for in the analysis. Third, while participants in the placebo group reported a significant reduction in pain intensity, we did not determine the proportion of participants who believed they were actually receiving morphine. This measure could be included in future studies in order to more fully describe the efficacy of placebo. Fourth, structural MRI does not have the ability to determine the mechanisms that underlie the observed morphological changes, especially as many cellular changes can manifest similarly on MRI scans [32,33]. Translational studies that aim to determine the cellular and molecular nature of these changes could increase our understanding of the underlying mechanisms of the morphological changes associated with short- and long-term opioid use. Finally, although many of the observed changes were considered to be likely consequences of morphine administration, we were not able to determine the behavioral implications of these changes.
The current findings corroborate previous reports that morphological changes occur rapidly in the human brain during new exposure to prescription opioid analgesics. Decreased gray matter volume in the amygdala and other reward-processing areas were seen after 1 month of daily morphine administration. Our previous and current study provides the impetus for further research into the neural correlates of pain and opioid analgesia. Future studies should include larger sample sizes and additional outcome measures with extensive behavioral, cognitive, and sensory tests, which may help to distinguish whether there are specific neural changes associated with beneficial outcomes (e.g., analgesia) and adverse consequences (e.g., cognitive impairment, addiction). Longitudinal studies can be designed to investigate the effects of prolonged opioid use, and those that include a follow-up of participants who have discontinued their prescription opioids may also help to characterize the reversibility of opioid-induced morphological changes. Future studies may also investigate the effects of opioids on the developing brain given the recent approval for the use of extended release oxycodone in certain pediatric populations [34].
Improving the understanding of the relationship between brain changes and functional outcomes may allow us to develop predictive models of risk for negative opioid effects and, ultimately, increase the clinical utility of opioids for chronic pain.
Funding sources: Dr. Younger was supported with a career development award from the National Institute on Drug Abuse (K99DA023609).
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.National Center for Health Statistics. Health, United States, 2013: With Special Feature on Prescription Drugs; Hyattsville, MD: 2014. [PubMed] [Google Scholar]
- 2.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med 2003;349:1943–53. [DOI] [PubMed] [Google Scholar]
- 3.Ersek M, Cherrier MM, Overman SS, Irving GA. The cognitive effects of opioids. Pain Manag Nurs 2004;5:75–93. [DOI] [PubMed] [Google Scholar]
- 4.Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: A preliminary prospective study. J Pain 2006;7:43–8. [DOI] [PubMed] [Google Scholar]
- 5.Moore P, Dimsdale JE. Opioids, sleep, and cancer-related fatigue. Med Hypotheses 2002;58:77–82. [DOI] [PubMed] [Google Scholar]
- 6.Glass MJ, Kruzich PJ, Colago EEO, Kreek MJ, Pickel VM. Increased AMPA GluR1 receptor subunit labeling on the plasma membrane of dendrites in the basolateral amygdala of rats self-administering morphine. Synapse 2005;58:1–12. [DOI] [PubMed] [Google Scholar]
- 7.Maher CE, Martin TJ, Childers SR. Mechanisms of mu opioid receptor/G-protein desensitization in brain by chronic heroin administration. Life Sci 2005;77:1140–54. [DOI] [PubMed] [Google Scholar]
- 8.Zarrindast M-R, Ahmadi S, Haeri-Rohani A, et al. GABAA receptors in the basolateral amygdala are involved in mediating morphine reward. Brain Res 2004;1006:49–58. [DOI] [PubMed] [Google Scholar]
- 9.Upadhyay J, Maleki N, Potter J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain 2010;133:2098–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: Relationships to drug addictions. Biochem Pharmacol 2008;75:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc B Biol Sci 2008;363:3113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Merrer J, Becker JAJ, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev 2009;89:1379–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younger JW, Chu LF, D’Arcy NT, et al. Prescription opioid analgesics rapidly change the human brain. Pain 2011;152:1803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129–38. [PubMed] [Google Scholar]
- 15.Cleeland CS. The Brief Pain Inventory: User Guide. 2009. http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/BPI_UserGuide.pdf (accessed September 21, 2015). [Google Scholar]
- 16.Leow AD, Klunder AD, Jack CR, et al. Longitudinal stability of MRI for mapping brain change using tensor-based morphometry. Neuroimage 2006;31:627–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashburner J, Andersson JLR, Friston KJ. Image registration using a symmetric prior—In three dimensions. Hum Brain Mapp 2000;9:212–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 19.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 2002;15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol 2005;210:343–52. [DOI] [PubMed] [Google Scholar]
- 21.Baumgärtner U, Buchholz H-G, Bellosevich A, et al. High opiate receptor binding potential in the human lateral pain system. Neuroimage 2006;30:692–9. [DOI] [PubMed] [Google Scholar]
- 22.Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci 2005;6:691–702. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Soelch C, Chevalley AF, Künig G, et al. Changes in reward-induced brain activation in opiate addicts. Eur J Neurosci 2001;14:1360–8. [DOI] [PubMed] [Google Scholar]
- 24.Lyoo IK, Pollack MH, Silveri MM, et al. Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology 2006;184:139–44. [DOI] [PubMed] [Google Scholar]
- 25.Daglish MR, Weinstein A, Malizia AL, et al. Functional connectivity analysis of the neural circuits of opiate craving: “more” rather than “different”? Neuroimage 2003;20:1964–70. [DOI] [PubMed] [Google Scholar]
- 26.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005;6:533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong J, White NS, Kwong KK, et al. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp 2006;27:715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra G, Coombes SA. Neuroimaging evidence of motor control and pain processing in the human midcingulate cortex. Cereb Cortex 2015;25:1906–19. [DOI] [PubMed] [Google Scholar]
- 29.Pfeiffer A, Pasi A, Mehraein P, Herz A. Opiate receptor binding sites in human brain. Brain Res 1982;248:87–96. [DOI] [PubMed] [Google Scholar]
- 30.Borsook D, Becerra L, Carlezon WA, et al. Reward-aversion circuitry in analgesia and pain: Implications for psychiatric disorders. Eur J Pain 2007;11:7. [DOI] [PubMed] [Google Scholar]
- 31.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci 2008;9:314–20. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson SH, Free SL, Thom M, et al. Quantitative grey matter histological measures do not correlate with grey matter probability values from in vivo MRI in the temporal lobe. J Neurosci Methods 2009;181:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. J Neurosci 2005;25:8303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Food and Drug Administration. CDER Conversation: Pediatric Pain Management Options. 2015. http://www.fda.gov/Drugs/NewsEvents/ucm456973.htm (accessed September 25, 2015). [Google Scholar]