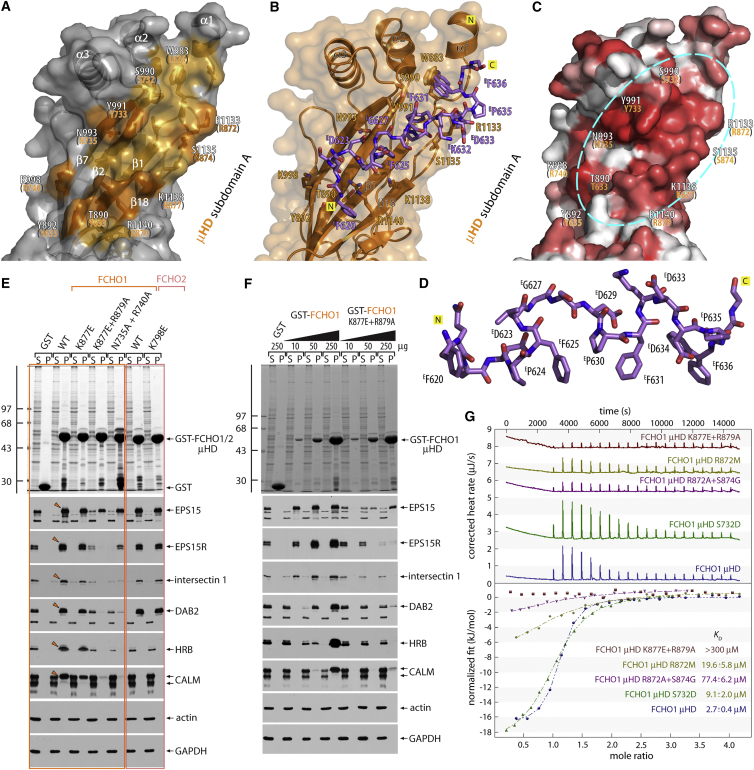
Figure 3.
A Major Interaction Surface on Fcho1 μHD Subdomain A
(A–C) Combination ribbon and molecular surface representation of the concave face of zebrafish Fcho1 μHD subdomain A showing the EPS15 binding trough. (A) The β strands involved and projecting μHD contact residues (light orange), determined by the PISA server. Selected side chains mutated are indicated (dark orange) with the corresponding residue number in the FCHO1 μHD indicated in orange type. (B) View of the tandem DFP motifs (stick representation with carbon mauve, nitrogen blue, and oxygen atoms red) bound to the μHD. EF636 is shown in a dual conformation with μHD W833 also in dual conformation to permit flipping of EF636. (C) ENDscript 2 (Robert and Gouet, 2014) computed phylogenetic surface conservation between muniscin μHDs using the TrEMBL (opisthokonta) database for sequence alignments. Conservation graded in shades from invariant (red) to unrelated (white) projected onto the solvent-accessible molecular surface of the Fcho1 μHD. The surface conservation reveals a patch (cyan oval) of invariant and highly conserved residues at the binding site on subdomain A, while there is a rather discombobulated patchwork pattern of conservation over the convex face.
(D) The bound DPF tract showing the spatial arrangement of the aromatic Phe and Pro side chains packing into the μHD trough.
(E) Pull-down assay utilizing HeLa cell lysate and 250 μg of GST, GST-FCHO1 (orange box), or GST-FCHO2 (pink box) μHD, or the indicated mutant. Stained gel and replicate blots were probed with the indicated antibodies. Position of bound partner protein (arrowheads) is indicated.
(F) Pull-down assay with HeLa cell lysate and the indicated amount of GST, GST-FCHO1 μHD, or K877E + R879A mutant immobilized on glutathione-Sepharose.
(G) Representative ITC experiments of wild-type FCHO1 or color-coded mutant μHDs binding to an EPS15 616–638 peptide.