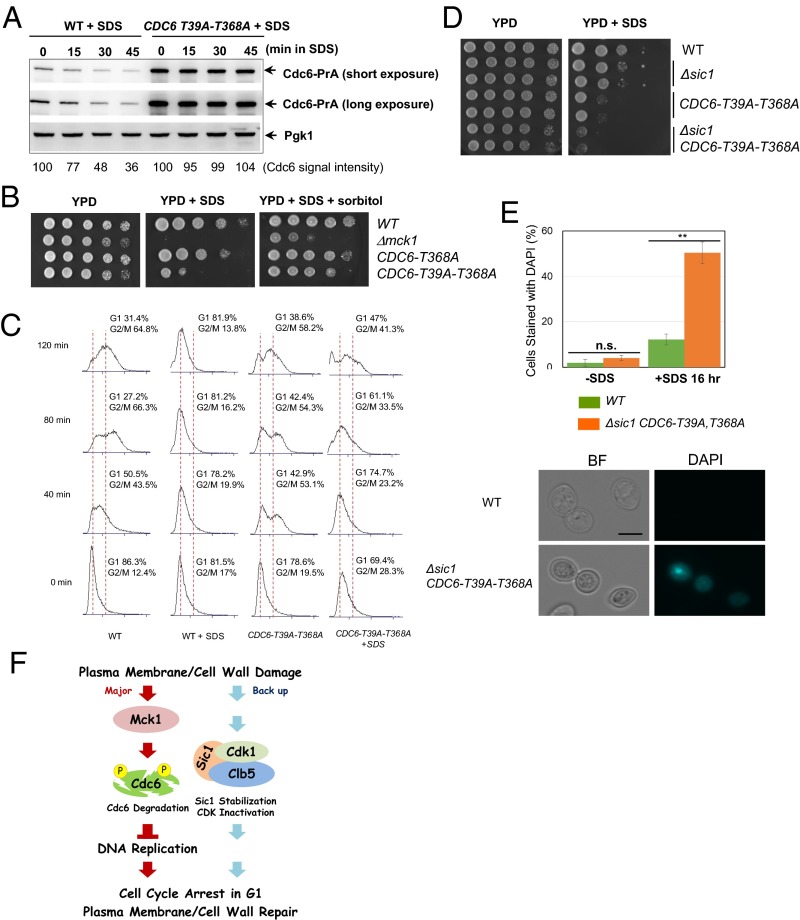
Fig. 4.
Phosphorylation of Cdc6-T39 and T368 is required for Cdc6 degradation and cell cycle arrest after plasma membrane damage. (A) CDC6-prA or CDC6-T39A-T368A-prA cells were grown to log-phase. SDS at 0.0075% concentration was added to the media, and samples were collected every 15 min. Protein was extracted from each time point for Western blotting to detect Cdc6-prA or Pgk1 as a loading control. (B) Indicated W303 background yeast strains were serially diluted at fivefold, and spotted on YPD, YPD containing 0.005% SDS, or YPD containing 0.005% SDS plus 1 M sorbitol plates. Plates were incubated for 2–3 days. (C) Indicated strains were grown in YPD and were synchronized in G1 phase by α-factor. The G1 block was released into nocodazole-containing YPD media plus 0.0075% SDS at time 0. Samples were collected every 40 min to monitor cell cycle progression via FACS analysis. (D) Indicated strains were serially diluted at 10-fold and plated on YPD with or without 0.0075% SDS plates. (E) Indicated strains were grown in YPD with or without 0.0075% SDS for 16 h. Cells were washed once with YPD and then stained with DAPI to visualize cells with a ruptured plasma membrane under a fluorescence microscope. Averages of three independent experiments are shown. n > 100 per each experiment. Error bars, SD **P < 0.01 (Student’s t test). (Scale bar, 5 μm.) The images are representations of DAPI stained cells treated with SDS. (F) A model of a novel cell cycle checkpoint activated by plasma membrane damage.