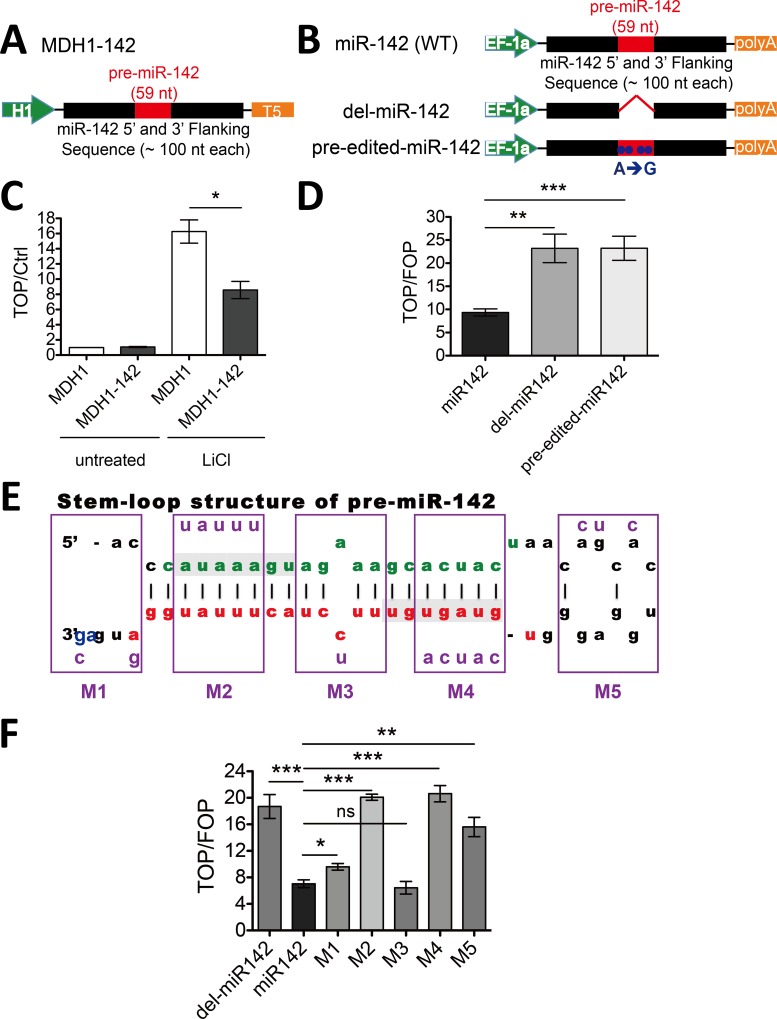
Fig 2. Pre-miR-142 is essential for suppressing Wnt/β-catenin signaling.
A and B, schematic of the MDH1-142 (A) and EF-miR-142, EF-del-miR-142, EF-pre-edited-miR-142 (B) constructs. Pre-miR-142 (59 nt) plus flanking sequences were introduced downstream of the H1 or EF-1a promoter, followed by T5 or polyA termination signal. Whole 59 nucleotides of pre-miR-142 were deleted in the del-miR-142 construction. Four Adenosine residues within pre-miR-142 were substituted by guanosine residues (A➔G) constructed the pre-edited-miR-142 plasmid. (C) HEK293T cells were transfected with MDH1-142 expression vector or control MDH1 vector along with the pGL3-TopFlash or pGL3 empty vector and the pRL-TK vector as a normalization control. Cells were treated with 25 mM LiCl and lysed 24 h later for dual-luciferase analysis. Normalized TopFlash values were further divided by normalized pGL3 control values; error bar mark the SEM (n = 3; *P < 0.05, t test). (D) HEK293T cells were transfected with EF-miR142 expression vector or miR-142-mutant vectors along with the pGL3-TopFlash or pGL3-FopFlash vector and the pRL-TK vector as a normalization control. Normalized TopFlash values were further divided by normalized FopFlash values; error bars mark the SEM (n = 3; ***P < 0.001, **P < 0.01, t test). (E) Schematic diagram showing the structure of pre-miR-142. The part highlighted in green indicates miR-142-5p, and in red shows miR-142-3p. Bases with light-gray background represent the seed sequences of these two distinct miRNAs. The frames above M1 to M5 mark the positions of five structure-changing mutants within pre-miR-142, purple bases within the frames indicate the mutant sequences. (F) TopFlash-mediated reporter assay was performed as described in (D) with M1 ~ M5 mutants; error bars mark the SEM (n = 3; ***P < 0.001, **P < 0.01, *P < 0.05, ns P > 0.05, t test).