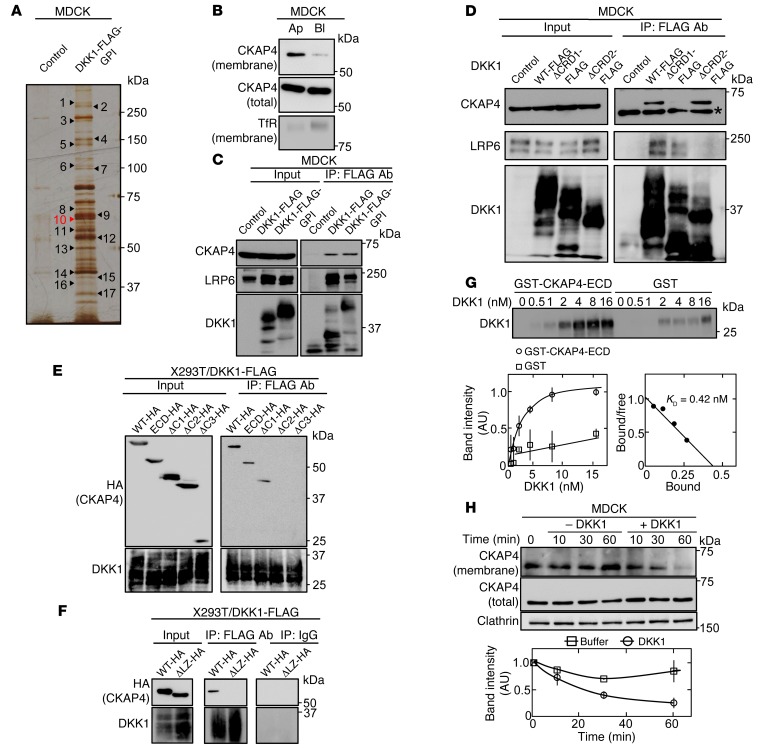
Figure 2. CKAP4 is identified as a novel DKK1-interacting protein.
(A) The DKK1-interacting proteins were detected by silver staining. Seventeen proteins that bind to DKK1-FLAG-GPI were analyzed by mass spectrometry. The results are listed in Supplemental Table 2. (B) Polarized MDCK cells were biotinylated apically (Ap) or basolaterally (Bl), and cell surface proteins were precipitated with NeutrAvidin Agarose beads. The precipitates were probed with anti-CKAP4 and anti–transferrin receptor (TfR) antibodies. TfR was used as a basolateral membrane protein marker. (C) Lysates (Input) of control MDCK, MDCK/DKK1-FLAG, or MDCK/DKK1-FLAG-GPI cells were immunoprecipitated with anti-FLAG antibody. The IPs were probed with the indicated antibodies. (D) Lysates (Input) of MDCK cells stably expressing WT or deletion mutants of DKK1-FLAG were immunoprecipitated with anti-FLAG antibody. The IPs were probed with the indicated antibodies. CRD, cysteine-rich domain. *Nonspecific band. (E and F) Various deletion mutants of CKAP4 were transiently expressed in X293T/DKK1-FLAG cells, and cell lysates were immunoprecipitated with anti-FLAG antibody or nonimmune IgG and the IPs were probed with the indicated antibodies. ECD, extracellular domain. (G) Top panel: The indicated concentration of DKK1 was incubated with 0.5 nM GST-CKAP4-ECD or 0.5 nM GST for 2 hours. The precipitates were probed with anti-DKK1 antibody. Bottom panels: The signals of DKK1 bound to GST-CKAP4-ECD (circles) or GST (squares) were quantified using ImageJ software and expressed as arbitrary units. Scatchard analysis of the binding is shown. (H) MDCK cells were stimulated with 10 nM DKK1, and cell surface proteins were biotinylated at each time point and precipitated with NeutrAvidin Agarose beads. The precipitates were probed with anti-CKAP4 and anti-clathrin antibodies. Clathrin was used as a loading control. Results are shown as means ± SD of 3 independent experiments.