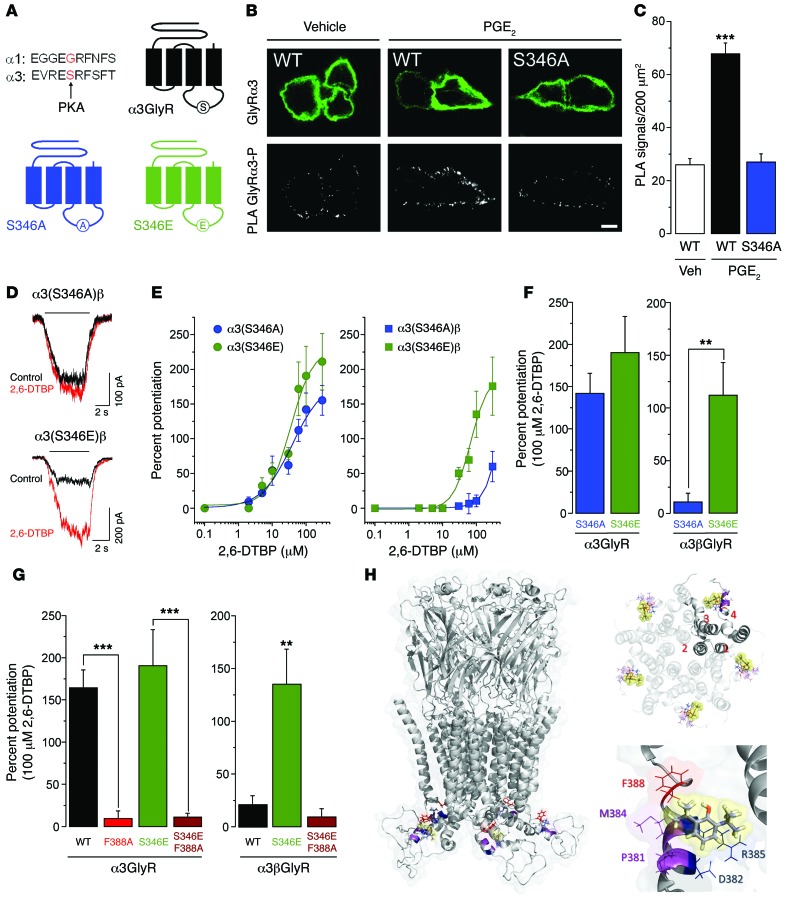
Figure 3. Influence of S346 phosphomutation in α3GlyR on allosteric modulation by 2,6-DTBP.
(A) Localization of the S346 residue in the primary sequence of the mouse GlyR α3 subunit, the corresponding sequence in the GlyR α1 subunit, and schematic diagram illustrating this site in the α3GlyR topology. (B) Proximity ligation assay (PLA) demonstrating phosphorylation of α3GlyR by PGE2 (1 μM) in HEK293T cells transfected with the EP2 receptor and wild-type GlyR α3 or (S346A) point-mutated GlyR α3 subunit plasmids. Top: α3GlyR immunofluorescence, bottom: PLA signal. Scale bar: 5 μm. (C) PGE2 significantly increased PLA signals in HEK293T cells transfected with wild-type but not with (S346A) GlyR α3 subunits. ***P < 0.001, ANOVA followed by Bonferroni post-hoc test, F(2,141) = 49.84; P < 0.001, PGE2 in wild-type α3GlyR-transfected cells versus the other two conditions. (D) Example traces of glycine-activated currents in α3(S346A)β or α3(S346E)βGlyRs in the absence and presence of 2,6-DTBP (100 μM). (E) Concentration-response curves for 2,6-DTBP in homomeric α3 (left) and heteromeric α3βGlyRs (right) containing S346E or S346A mutations. (F) The phosphomimicking S346E mutation restored 2,6-DTBP sensitivity of α3βGlyRs but had little effect on that of homomeric α3GlyRs. **P < 0.01, unpaired t test. (G) The F388A point mutation in α3GlyRs abolished 2,6-DTBP sensitivity. Introducing the S346E mutation did not restore 2,6-DTBP sensitivity in homomeric α3(F388A)GlyRs or heteromeric α3(F388A)βGlyRs. **P < 0.01, ***P < 0.001, ANOVA followed by Bonferroni post-hoc test, F(3,35) = 20.7 (left) and F(2,16) = 8.81 (right). (H) Structural model of the α3GlyR. Left: location of the F388 residue (red) and of 2,6-DTBP (yellow sphere) in a homopentameric α3GlyR. Other relevant residues are shown in magenta (P381 and M384) and blue (D382 and R385). Top, right: view from the middle of the plasma membrane on the putative acceptor sites with 2,6-DTBP bound (in yellow spheres) within a pentameric complex. Bottom, right: detailed view of the putative acceptor site of 2,6-DTBP. The hydrophobic interaction supported by F388 is highlighted in red.