Abstract
Objective
The aim of this study was to investigate age-related variations in the effect of body mass index (BMI) on in vitro fertilization (IVF) outcomes.
Material and Methods
This was a cohort study conducted by retrospectively investigating the IVF cycles of 653 polycystic ovary syndrome (PCOS) patients under the age of 40 years who were diagnosed based on the Rotterdam criteria in a private IVF clinic between 2005 and 2015. The study included data from 653 IVF cycles of PCOS patients. The patients were classified into three groups based on their BMI, i.e., normal weight (n=299), overweight (n=208), and obese (n=146). The patients were also grouped by age: 562 patients were under the age of 35 years and 91 patients were above the age of 35 years. Then, BMI- and age-related variations in the IVF cycle parameters and clinical pregnancy rates of patients with PCOS were investigated. The Mantel–Haenszel Chi-square statistical assessment method was used to determine whether the effect of BMI on IVF outcomes varies with age.
Results
Variations in cycle variables with BMI and age showed that IVF cycles were negatively affected by increases in obesity and age. Clinical pregnancy rates were found to be lower in the obese group than in the other groups, particularly in the age group above 35 years; however, this difference could not be proven statistically.
Conclusion
The present study evaluated obesity and clinical pregnancy rates in IVF cycles in PCOS patients according to age groups, and particularly in the obese group, the clinical pregnancy rates were observed to be lower in the age group ≥35 years than in the other BMI groups; however, this difference was found to be statistically insignificant.
Keywords: PCOS, body mass index, age, IVF outcome
Introduction
There are numerous potential causes of female infertility, and a systematic approach is necessary to effectively identify them in patients. Polycystic ovary syndrome (PCOS) is the most frequently observed cause of treatable infertility. It is commonly encountered among young women and accounts for nearly 70% of cases involving anovulatory infertility (1). In vitro fertilization (IVF) is an option that is recommended for infertility with no response to medical treatment in PCOS (2).
Nearly half of all women with PCOS are either obese or overweight, and most exhibit the abdominal phenotype (3). Recently, Lindsay et al. (4) showed that weight loss has a corrective effect on reproductive outcomes in all obese infertile patients. In 2015, Carmina et al. (5) demonstrated that weight loss increased spontaneous fertility rates in anovulatory PCOS patients.
The literature contains different opinions about the role of body mass index (BMI) in IVF cycles. A study by Loveland et al. (6) demonstrated that implantation rates and pregnancy rates decrease with increasing BMI. In 2006, Dechaud et al. (7) showed that obesity does not have any adverse impact on IVF, and Metwally et al. (8) showed that obesity does not affect oocyte quality or clinical pregnancy rates in IVF/intracytoplasmic sperm injection (ICSI) cycles in 2007.
Because weight loss in PCOS has positive effects on hormonal, metabolic, and clinical parameters, recent studies have focused on the effect of BMI during IVF cycles in PCOS patients. Studies of IVF in PCOS also suggest different opinions about BMI: Fedorcsák et al. (9) and Mulders et al. (10) demonstrated that obese women with PCOS show gonadotropin resistance in IVF cycles; therefore, the need for follicle-stimulating hormone (FSH) and cycle cancellations increases. A study by McCormick et al. (11) demonstrated that patients with PCOS who, according to their BMI, were leaner rather than obese exhibited more positive responses to assisted reproductive technology cycles; however, these patients also exhibited no remarkable differences with respect to clinical outcomes. In 2014, Bailey et al. (12) investigated the effect of BMI on the characteristics and outcomes of IVF cycles in PCOS and found lower clinical pregnancy rates in an obese PCOS group than in a lean group and a lower rate of ovarian hyperstimulation syndrome (OHSS) in the obese group.
A woman’s age is also an important factor in IVF success (13, 14). The IVF success rate reduces with increasing age in PCOS, as in all infertile patient groups (15, 16).
The literature contains a very limited number of studies that investigate age and BMI together. Heijnen et al. (17) reported that although BMI has a significant and negative effect on female fertility, this effect gradually decreases as women approach their mid-thirties and also in younger women receiving IVF. Above the age of 36 years, on the other hand, the effect of BMI on fertility becomes minimal. PCOS patients were not included in this study. The study by Metwally et al. (8) that investigated 426 IVF/ICSI cycles did not exclude PCOS patients and reported that although obesity does not affect oocyte quality, there is a reduction in embryo quality in patients under the age of 35 years.
The hypothesis of the present study assumed that the effect of BMI on the IVF cycles of PCOS patients may vary with age. The present study aimed to investigate age-related variations in the effect of BMI on IVF outcomes.
Material and Methods
This was a cohort study conducted by retrospectively investigating the IVF cycles of 653 PCOS patients under the age of 40 years, who were diagnosed based on the Rotterdam criteria in Gürgan IVF clinic between 2005 and 2015. The study protocol was approved by the local ethics committee, and all patients included in the study provided informed consent.
The patients were diagnosed with PCOS in accordance with the diagnostic criteria of the European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine Rotterdam Consensus Meeting 2003 (18). Based on this set of criteria, two of the following three criteria are necessary for confirming a diagnosis:
- Anovulation or Oligoovulation
- Biochemical or Clinical Hyperandrogenism
- Polycystic ovaries identified by ultrasound examination
Weight and height information was obtained from the patient database, and the following formula was used to calculate the BMI value: BMI=weight/height2 (kg/m2). Patient classification was performed based on BMI, with the patients being divided into three groups according to the World Health Organization’s (WHO’s) system for obesity (WHO, 2000). As such, normal weight was defined as a BMI value between 18.5 and 24.9 kg/m2; overweight was defined as a BMI value between 25 and 29.9 kg/m2; obese was defined as a BMI value of ≥30 kg/m2 (19).
Patients were also classified into two groups based on age, i.e., under and above the age of 35 years (Table 1).
Table 1.
Number of patients by BMI and age
| BMI (kg/m2) | n | Age (years) | n |
|---|---|---|---|
| Normal weight | 299 | ||
| Overweight | 208 | <35 | 562 |
| Obese | 146 | ≥35 | 91 |
| Total | 653 | Total | 653 |
BMI: body mass index
Female patients above 40 years of age, with basal FSH levels of >12 IU/L, and with frozen embryo transfer cycles were excluded from the study. Couples with systemic diseases such as diabetes and hypo- or hyperthyroidism, psychiatric diseases, and drug use in either the woman or the man were excluded. Although it was planned to have patients with a BMI of <18.5 to form a low-weight group, the number of patients in this group was very low; therefore, patients with a BMI of <18.5 were excluded.
In all patients, controlled ovarian hyperstimulation (COH) and (ICSI were performed using a classic ovulation induction protocol. For COH, long agonist and antagonist protocols were applied; gonadotropins were administered using step-up, step-down, and constant regimens. Ovulation induction was performed using urinary FSH (Metrodin; Serono, Geneva, Switzerland), recombinant FSH (Gonal F; Serono, Geneva, Switzerland or Puregon; Organon, Oss, The Netherlands), or urinary and recombinant FSH, and ovulation stimulation was performed using human chorionic gonadotropin (hCG) (Pregnyl; Organon, Brussels, Belgium).
The cycle parameters that were evaluated for this study included the protocols used in ovulation induction, total drug dose, ovulation induction time, cycle day of hCG, number of follicles and estradiol levels on the hCG day, endometrial thickness on the hCG day, number of oocytes retrieved, number of mature oocytes, number of embryos developed, number of grade 1 embryos, and number of embryos transferred. The IVF outcome parameters were the presence/absence of clinical pregnancy and multiple pregnancy rates. Clinical pregnancy was diagnosed by the embryo reaching the end of week 6, along with a proper image of the gestational sac, and the establishment of regular rhythmic heartbeats.
In the statistical assessment, firstly the effects of BMI and age on the cycle parameters and the rates of clinical pregnancy achieved were evaluated separately. Bivariate analyses such as the Mann–Whitney U test, Chi-square test, and/or Fisher’s exact test were used to evaluate group comparisons and results were summarized as median (minimum–maximum) and frequencies, where appropriate. In addition, the Mantel–Haenszel Chi-square statistical assessment method was used to determine whether the effect of BMI on IVF outcomes varies with age. Values of p less than 0.05 were considered to be significant. SPSS for Windows 11.5 (SPSS Inc.; Chicago, IL, USA) was used for statistical analysis.
Results
The study included data from 653 IVF cycles of PCOS patients. The patients were classified into three groups based on their BMI, i.e., normal weight (n=299), overweight (n=208), and obese (n=146). When the patients were grouped by age, 562 patients were under the age of 35 years and 91 patients were above the age of 35 years (Table 1).
The demographic characteristics of the couples included in the study are summarized in Table 2.
Table 2.
Demographic characteristics of the couples included in the study
| Parameter | Mean±SD | Median (min.–max.) |
|---|---|---|
| Female | ||
| Age | 29.30±4.81 | 29.00 (16.00–39.00) |
| BMI (kg/m2) | 26.45±5.12 | 25.59 (11.75–46.65) |
| FSH (IU/L) | 3.57±1.83 | 3.35 (0.10–19.00) |
| LH (IU/L) | 1.44±0.99 | 1.69 (0.10–2.30) |
| E2 (pg/mL) | 47.98±190.01 | 23.00 (8.00–2899.00) |
| Male | ||
| Age (years) | 33.58±5.52 | 33 (13–65) |
| Sperm count | 39761455±45713180 | 36000000 (12–910000000) |
| Motility (%) | 55.91±15.29 | 58.00 (2.00–93.00) |
| Progression (%) | 22.31±9.51 | 23.00 (1.00–50.00) |
BMI: body mass index; FSH: follicle-stimulating hormone; LH: luteinizing hormone; E2: estradiol
Details obtained from an examination of the male factor in the couple are presented in Table 3.
Table 3.
Results of sperm analysis
| Sperm analysis | Frequency | Percentage |
|---|---|---|
| Normospermia | 193 | 29.6 |
| Teratozoospermia | 178 | 27.3 |
| Oligoasthenoteratospermia | 137 | 21.0 |
| Asthenoteratozoospermia | 73 | 11.2 |
| Azoospermia | 68 | 10.4 |
| Immotile sperm | 4 | 0.6 |
| TOTAL | 653 | 100.0 |
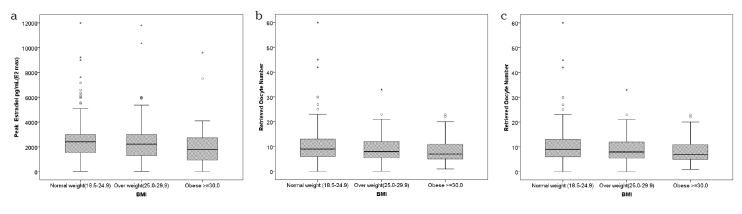
The data obtained from comparing the cycle parameters according to the BMI groups are summarized in Table 4. No difference was found between the groups in the distribution of induction protocols, ovulation induction times, and total drug doses (p=0.320, 0.180, and 0.298, respectively). There was no statistically significant difference in the total number of follicles on the hCG day, endometrial thickness, embryo transfer days, number of embryos developed, number of grade 1 embryos, and number of embryos transferred between all three groups (p=0.846, 0.529, 0.886, 0.176, 0.833, and 0.639, respectively). Of the cycle parameters, the maximum estradiol (E2) levels, number of oocytes retrieved, and number of mature oocytes were found to be significantly lower in the obese group than in the normal weight group (p=0.007, 0.001, and 0.047, respectively) (Figure 1). The rates of OHSS, clinical pregnancy, and multiple pregnancy were not different between the groups (p=0.933, 0.129, and 0.121, respectively).
Table 4.
Comparison of IVF cycle parameters in PCOS patients according to BMI group
| Parameter | BMI | |||
|---|---|---|---|---|
| Normal weight BMI (18.5–24.9) | Overweight BMI (24.9–29.9) | Obese BMI ≥30 | p | |
| GnRH treatment (long agonist protocol) | 149 (58.9%) | 100 (59.5%) | 59 (51.8%) | 0.367 |
| Duration of GnRH treatment (days) | 24.0 (20.0–44.0) | 24.0 (21.0–32.0) | 24.0 (20.0–36.0) | 0.123 |
| Protocols of OI | ||||
| Step-down | 178 (66.9%) | 111 (60%) | 74 (57.8%) | 0.320 |
| Step-up | 36 (13.5%) | 34 (18.4%) | 21 (16.4%) | |
| Constant | 52 (19.5%) | 40 (21.6%) | 33 (25.8%) | |
| E2 max (pg/mL) | 2411 (43–11985)a | 2229 (38–11800) | 1796 (19–9600)b | 0.007* |
| Number of retrieved oocytes | 9 (0–60)a | 8 (0–33)ab | 7 (1–23)b | 0.001* |
| Number of mature oocytes | 7 (0–55)a | 7 (0–30)ab | 6 (0–21)c | 0.047* |
| Duration of OI (days) | 8 (0–28) | 9 (0–27) | 9 (0–24) | 0.180 |
| Total dose of FSH (IU) | 2550 (0–3150) | 2887 (0–3075) | 3375 (0–5625) | 0.298 |
| hCG day | 12 (7–32) | 13 (6–30) | 12 (7–24) | 0.007* |
| ≥17 mm follicle on hCG day | 1 (0–9) | 1 (0–8) | 1 (0–7) | 0.846 |
| 15–17 mm follicle on hCG day | 4 (0–14) | 4 (0–14) | 3 (0–12) | 0.342 |
| 10–14 mm follicle on hCG day | 9 (0–35) | 9 (0–41) | 8 (0–28) | 0.587 |
| Endometrial thickness on hCG day (mm) | 9.9 (0–14.5) | 9.9 (5–13.8) | 9.5 (0–14.3) | 0.529 |
| Day of transfer | 0.886 | |||
| D2 | 33 (45.8%) | 22 (30.6%) | 17 (23.6%) | |
| D3 | 202 (44.3%) | 152 (33.3%) | 102 (22.4%) | |
| D4 | 19 (52.8%) | 12 (33.3%) | 5 (13.9%) | |
| D5 | 30 (44.1%) | 19 (27.9%) | 19 (27.9%) | |
| D6 | 3 (60%) | 1 (20%) | 1 (20%) | |
| Number of embryos | 6 (0–25) | 5 (0–24) | 5 (0–21) | 0.176 |
| Number of grade 1 embryos | 2 (0–8) | 2 (0–9) | 2 (0–8) | 0.639 |
| Number of grade 1 embryos (Frag0+Frag<10%) | 2 (0–13) | 2 (0–13) | 2 (0–19) | 0.833 |
| OHSS | 5 (1.7%) | 4 (1.9%) | 3 (2.1%) | 0.933 |
| Clinical pregnancy | 101 (33.7%) | 78 (37.5%) | 40 (27.3%) | 0.129 |
| Multiple pregnancy | 48 (53.3%) | 37 (50%) | 12 (33.3%) | 0.121 |
A value of p<0.05 is statistically significant.
IVF: in vitro fertilization; PCOS: polycystic ovary syndrome; BMI: body mass index; GnRH: gonadotropin-releasing hormone; OI: ovulation induction; D: day of embryo; E2 max: peak estradiol level; FSH: follicle-stimulating hormone; hCG: human chorionic gonadotropin; OHSS: ovarian hyperstimulation syndrome
0 cells (0%) expected count less than 5. The minimum expected count is 0.
Computed only for a 2×2 table.
The standardized statistic is 1.330.
Figure 1.
a–c. Distribution of maximum E2 levels (a), number of oocytes retrieved (b), and number of mature oocytes (c), according to BMI group
When the same parameters were compared between the group under the age of 35 years and the group aged 35 years and above, there was a difference in favor of the group aged 35 years and above only in the number of embryos transferred (p<0.001) (Figure 2). The number of embryos developed, number of grade 1 embryos, clinical pregnancy rates, and OHSS rates were higher in the group aged under 35 years; however, this was not statistically significant (Table 5).
Figure 2.
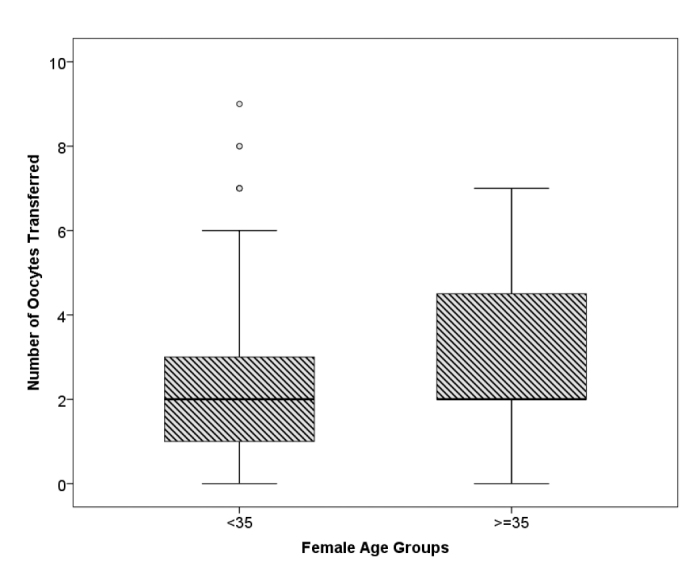
Distribution of number of embryos transferred (etemb) according to age group
Table 5.
Comparison of IVF cycle parameters in PCOS patients according to age group
| Parameter | Female Age | ||
|---|---|---|---|
| <35 | ≥35 | p | |
| GnRH treatment (long agonist protocol) | 262 (57.2%) | 46 (59.7%) | 0.677 |
| Duration of GnRH treatment (days) | 24 (20–37) | 24 (21–44) | 0.782 |
| Protocols of OI | |||
| Step-down | 395 (61.7%) | 58 (68.2%) | 0.527 |
| Step-up | 89 (16.2%) | 11 (12.9%) | |
| Constant | 109 (22.1%) | 18 (18.8%) | |
| Peak estradiol (pg/mL) (E2 max) | 2221 (38–11985) | 2316 (19–5900) | 0.620 |
| Number of retrieved oocytes | 9 (0–60) | 8 (0–21) | 0.189 |
| Number of mature oocytes | 7 (0–55) | 6 (0–19) | 0.390 |
| Duration of OI (days) | 8 (0–27) | 9 (0–28) | 0.156 |
| Total dose of FSH (IU) | 2925 (0–3150) | 3525 (0–5625) | 0.134 |
| hCG day | 12 (7–30) | 12 (6–32) | 0.481 |
| ≥17 mm follicle on hCG day | 1 (0–9) | 1 (0–6) | 0.809 |
| 15–17 mm follicle on hCG day | 4 (0–14) | 4 (0–12) | 0.836 |
| 10–14 mm follicle on hCG day | 9 (0–41) | 7.5 (0–30) | 0.291 |
| Endometrial thickness on hCG day (mm) | 9.9 (0–14.5) | 9.6 (4.6–14) | 0.350 |
| Day of transfer | 0.735 | ||
| D2 | 60 (10.9%) | 12 (13.5%) | |
| D3 | 392 (71.5%) | 64 (71.9%) | |
| D4 | 33 (6%) | 3 (3.4%) | |
| D5 | 59 (10.8%) | 9 (10.1%) | |
| D6 | 4 (0.7%) | 1 (1.1%) | |
| Number of embryos | 5 (0–25) | 5 (0–15) | 0.530 |
| Number of transferred embryos | 2 (0–9) | 2 (0–7) | <0.001* |
| Number of grade 1 embryos (Frag0+Frag<10%) | 2 (0–19) | 2 (0–11) | 0.111 |
| OHSS | 10 (1.8%) | 2 (2.2%) | 0.678 |
| Clinical pregnancy | 193 (34.3%) | 26 (28.5%) | 0.140 |
| Multiple pregnancy | 83 (47.2%) | 14 (58.3%) | 0.304 |
A value of p<0.05 is statistically significant.
IVF: in vitro fertilization; PCOS: polycystic ovary syndrome; GnRH: gonadotropin-releasing hormone; OI: ovulation induction; D: day of embryo; E2 max: peak estradiol level; FSH: follicle-stimulating hormone; hCG: human chorionic gonadotropin; OHSS: ovarian hyperstimulation syndrome
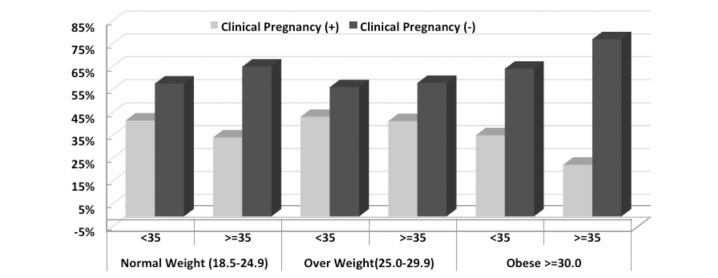
The obesity and clinical pregnancy rates in PCOS patients were evaluated according to age groups, and particularly in the obese group, the clinical pregnancy rates were observed to be lower in the group aged ≥35 years than in the other BMI groups; however, this difference was analyzed using Mantel–Haenszel Chi-square statistics and found to be statistically insignificant (Mantel–Haenszel Chi-square statistic=1.34, p=0.247) (Table 6, Figure 3).
Table 6.
Evaluation of obesity and clinical pregnancy rates according to age group in PCOS patients
| BMI | Female Age n (%) | |||
|---|---|---|---|---|
| <35 | ≥35 | |||
| Normal weight (18.5–24.9) | Clinical pregnancy | Yes | 92 (42%) | 9 (34.6%) |
| Overweight (25.0–29.9) | Clinical pregnancy | Yes | 68 (43.6%) | 10 (41.7%) |
| Obese ≥30.0 | Clinical pregnancy | Yes | 33 (35.5%) | 7 (22.6%) |
PCOS: polycystic ovary syndrome; BMI: body mass index
Figure 3.
Evaluation of obesity and clinical pregnancy rates according to age group in PCOS patients
Discussion
The present study investigated the BMI- and age-related variations in the IVF cycle parameters and clinical pregnancy rates of patients with PCOS. The variations in cycle variables with BMI and age showed that the IVF cycles were negatively affected by increases in obesity and age. The clinical pregnancy rates were found to be lower in the obese group than in the other groups, particularly in the age group above 35 years; however, this difference could not be proven statistically.
In the first part of the present study, in which the relationship of the cycle parameters with BMI in IVF cycles in PCOS patients was investigated, there were reductions in the number of oocytes retrieved, maximum E2 levels, and number of mature oocytes in the obese group compared with the normal weight group (p=0.001, 0.007, and 0.047, respectively), but no significant difference was found in other cycle parameters between BMI groups.
The literature contains several studies that report that oocyte maturation is impaired in PCOS (20), oocyte quality and fertilization capacities are reduced (21), and, in addition, obesity contributes to such impaired maturation in PCOS (22–24). Fedorcsák et al. (9) reported that the number of oocytes retrieved was low in PCOS patients. A study by Robker et al. (25) with PCOS subgroups reported that the number of oocytes retrieved and the fertilization rates were lower in an obese group. The results of the present study seemed to be consistent with these literature data.
Regarding the age-related variations in the cycle characteristics, when the same parameters were compared between the group under the age of 35 years and the group aged 35 years and above, there was a difference in favor of the group aged 35 years and above only in the number of embryos transferred (p<0.001) (Figure 2). The number of embryos developed, number of grade 1 embryos, clinical pregnancy rates, and OHSS rates were higher in the group aged under 35 years; however, this was not statistically significant. The higher number of embryos transferred in the group aged 35 years and above is a natural outcome of transferring more embryos at advanced ages. Whether in PCOS patients, it is a generally accepted fact in the literature that advanced maternal age has a negative effect on all parameters in IVF cycles (14, 26–29). In the present study, although a statistically significant difference could not be demonstrated, it was seen that all parameters were negatively affected in the group aged 35 years and above.
The present study evaluated obesity and clinical pregnancy rates in IVF cycles in PCOS patients according to age group, and particularly in the obese group, the clinical pregnancy rates were observed to be lower in the age group aged ≥35 years than in the other BMI groups; however, this difference was found to be statistically insignificant.
Considering studies regarding BMI in IVF cycles in PCOS patients, a study by Bailey et al. (12), which specifically compared BMI and clinical pregnancy rates in PCOS patients, and in a similar manner to the data of the present study, showed a reduction in the clinical pregnancy rates in obese PCOS patients compared with thin PCOS patients.
A retrospective study by Gorelick et al. (31), which investigated 5208 IVF cycles with different diagnoses, included 439 cycles in PCOS patients and demonstrated that obesity did not have any effect on IVF outcomes in other groups, whereas among PCOS patients, obesity led to a twofold increase in the risk of failure in IVF treatment. In women with PCOS, various negative effects were identified, not only in the implantation rate but also in pregnancy and live birth outcomes (30).
McCormick et al. (11) previously conducted a study comparing obese and lean women with PCOS with obese and lean women without PCOS. The results of this study showed a higher number of retrievable oocytes among lean women with PCOS than among obese women with PCOS. However, the study also reported similar rates of clinical pregnancy and live birth between all groups. It is important to note that this study had a very small and limited sample size, with only six lean patients with PCOS included.
The data from the present study are in line with those from the three major studies mentioned above. No study could be identified in the literature that investigated the variation in the adverse effects of obesity on the IVF cycle data in PCOS patients with age. A study of IVF published in 2011 by Luke et al. (32) evaluated the age-related effect of obesity on IVF. This study analyzed the data of 45,000 embryo transfers and concluded that higher obesity levels (i.e., BMI values) resulted in a considerable increase in the inability to achieve clinical pregnancy via the use of autologous oocytes but resulted in no differences in the use of donor oocytes. Besides, it reported that the adverse effects of obesity were more evident in the group aged under 35 years (31). The present study observed the negative effects of obesity more specifically in the group aged above 35 years. This discrepancy is because the present study included only PCOS patients in the study group. In addition, the comparison in the abovementioned study was made between autologous and donor oocytes, which made it difficult to compare with the present study.
We believe that this study is the first to investigate age-related variations in the effects of BMI on IVF cycles in PCOS patients. The most important limitation of the present study is its retrospective nature. The retrospective examination of data from the last 10 years provided a higher number of cycles but prevented a statistically significant result from being achieved as it increased the heterogeneity of the data.
In conclusion, the present study evaluated the obesity and clinical pregnancy rates in IVF cycles in PCOS patients according to age groups, and particularly in the obese group, the clinical pregnancy rates were observed to be lower in the age group aged ≥35 years than in the other BMI groups; however, this difference was found to be statistically insignificant. Therefore, well-conceived, randomized controlled studies are required, which would investigate the effect of BMI and age on each other in PCOS patients, create a homogeneous and a higher number of subgroups, and include a larger patient population.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Turgut Özal University School of Medicine.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: M.N.K., Z.K.; Design - M.N.K., Z.K.; Supervision - T.G.; Materials - M.N.K., Z.K., T.G.; Data Collection and/or Processing - Z.K., T.S.; Analysis and/or Interpretation - M.N.K., C.A.; Literature Review - M.N.K., Z.K.; Writer - M.N.K., Z.K.; Critical Review - M.N.K., T.G.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Brassard M, AinMelk Y, Baillargeon JP. Basic infertility including polycystic ovary syndrome. Med Clin of North Am. 2008;92:1163–92. doi: 10.1016/j.mcna.2008.04.008. http://dx.doi.org/10.1016/j.mcna.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89:505–22. doi: 10.1016/j.fertnstert.2007.09.041. http://dx.doi.org/10.1016/j.fertnstert.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 3.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. http://dx.doi.org/10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Lindsay TJ, Vitrikas KR. Evaluation and treatment of infertility. Am Fam Physician. 2015;91 [PubMed] [Google Scholar]
- 5.Carmina E. Reproductive System Outcome Among Patients with Polycystic Ovarian Syndrome. Endocrinol Metab Clin North Am. 2015;44:787–97. doi: 10.1016/j.ecl.2015.07.006. http://dx.doi.org/10.1016/j.ecl.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Loveland JB, McClamrock HD, Malinow AM, Sharara FI. Clinical Assisted Reproduction: Increased Body Mass Index Has a Deleterious Effect on In Vitro Fertilization Outcome. J Assist Reprod Genet. 2001;18:382–6. doi: 10.1023/A:1016622506479. http://dx.doi.org/10.1023/A:1016622506479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dechaud H, Anahory T, Reyftmann L, Loup V, Hamamah S, Hedon B. Obesity does not adversely affect results in patients who are undergoing in vitro fertilization and embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2006;127:88–93. doi: 10.1016/j.ejogrb.2005.12.009. http://dx.doi.org/10.1016/j.ejogrb.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15:532–8. doi: 10.1016/s1472-6483(10)60385-9. http://dx.doi.org/10.1016/S1472-6483(10)60385-9. [DOI] [PubMed] [Google Scholar]
- 9.Fedorcsák P, Dale PO, Storeng R, Tanbo T, Åbyholm T. The impact of obesity and insulin resistance on the outcome of IVF or ICSI in women with polycystic ovarian syndrome. Hum Reprod. 2001;16:1086–91. doi: 10.1093/humrep/16.6.1086. http://dx.doi.org/10.1093/humrep/16.6.1086. [DOI] [PubMed] [Google Scholar]
- 10.Mulders AG, Laven JS, Imani B, Eijkemans MJ, Fauser BC. IVF outcome in anovulatory infertility (WHO group 2)–including polycystic ovary syndrome–following previous unsuccessful ovulation induction. Reprod Biomed Online. 2003;7:50–8. doi: 10.1016/s1472-6483(10)61728-2. http://dx.doi.org/10.1016/S1472-6483(10)61728-2. [DOI] [PubMed] [Google Scholar]
- 11.McCormick B, Thomas M, Maxwell R, Williams D, Aubuchon M. Effects of polycystic ovarian syndrome on in vitro fertilization-embryo transfer outcomes are influenced by body mass index. Fertil Steril. 2008;90:2304–9. doi: 10.1016/j.fertnstert.2007.10.077. http://dx.doi.org/10.1016/j.fertnstert.2007.10.077. [DOI] [PubMed] [Google Scholar]
- 12.Bailey AP, Hawkins LK, Missmer SA, Correia KF, Yanushpolsky EH. Effect of body mass index on in vitro fertilization outcomes in women with polycystic ovary syndrome. Am J Obstet Gynecol. 2014;211:163–e1. doi: 10.1016/j.ajog.2014.03.035. http://dx.doi.org/10.1016/j.ajog.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Dhillon RK, McLernon DJ, Smith PP, Fishel S, Dowell K, Deeks JJ, et al. Predicting the chance of live birth for women undergoing IVF: a novel pretreatment counselling tool. Human Reprod. 2016;31:84–92. doi: 10.1093/humrep/dev268. http://dx.doi.org/10.1093/humrep/dev268. [DOI] [PubMed] [Google Scholar]
- 14.Habbema JDF, Eijkemans MJ, Leridon H, te Velde ER. Realizing a desired family size: when should couples start? Human Reprod. 2015;30:2215–21. doi: 10.1093/humrep/dev148. http://dx.doi.org/10.1093/humrep/dev148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor TH, Patrick JL, Gitlin SA, Crain JL, Wilson JM, Griffin DK. Blastocyst euploidy and implantation rates in a young (<35 years) and old (≥35 years) presumed fertile and infertile patient population. Fertil Steril. 2014;102:1318–23. doi: 10.1016/j.fertnstert.2014.07.1207. http://dx.doi.org/10.1016/j.fertnstert.2014.07.1207. [DOI] [PubMed] [Google Scholar]
- 16.Boomsma CM, Eijkemans MJC, Hughes EG, Visser GHA, Fauser BCJM, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–83. doi: 10.1093/humupd/dml036. http://dx.doi.org/10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 17.Heijnen EMEW, Eijkemans MJC, Hughes EG, Laven JSE, Macklon N, Fauser BCJM. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:13–21. doi: 10.1093/humupd/dmi036. http://dx.doi.org/10.1093/humupd/dmi036. [DOI] [PubMed] [Google Scholar]
- 18.Sneed ML, Uhler ML, Grotjan HE, Rapisarda JJ, Lederer KJ, Beltsos AN. Body mass index: impact on IVF success appears age-related. Hum Reprod. 2008;23:1835–9. doi: 10.1093/humrep/den188. http://dx.doi.org/10.1093/humrep/den188. [DOI] [PubMed] [Google Scholar]
- 19.The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. http://dx.doi.org/10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Obesity: preventing and managing the global epidemic. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 21.Qiao J, Feng HL. Extra-and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17:17–33. doi: 10.1093/humupd/dmq032. http://dx.doi.org/10.1093/humupd/dmq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludwig M, Finas DF, Al-Hasani S, Diedrich K, Ortmann O. Oocyte quality and treatment outcome in intracytoplasmic sperm injection cycles of polycystic ovarian syndrome patients. Hum Reprod. 1999;14:354–8. doi: 10.1093/humrep/14.2.354. http://dx.doi.org/10.1093/humrep/14.2.354. [DOI] [PubMed] [Google Scholar]
- 23.Robker RL, Norman R. Obesity and oocyte quality. Biology and Pathology of the Oocyte: Role in Fertility. Medicine and Nuclear Reprograming. 2013:362. http://dx.doi.org/10.1017/CBO9781139135030.032. [Google Scholar]
- 24.Marquard KL, Stephens SM, Jungheim ES, Ratts VS, Odem RR, Lanzendorf S, et al. Polycystic ovary syndrome and maternal obesity affect oocyte size in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2011;95:2146–9. doi: 10.1016/j.fertnstert.2010.10.026. http://dx.doi.org/10.1016/j.fertnstert.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robker RL. Evidence that obesity alters the quality of oocytes and embryos. Pathophysiology. 2008;15:115–21. doi: 10.1016/j.pathophys.2008.04.004. http://dx.doi.org/10.1016/j.pathophys.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Cano F, García-Velasco JA, Millet A, Remohí J, Simón C, Pellicer A. Oocyte quality in polycystic ovaries revisited: identification of a particular subgroup of women. J Assist Reprod Genet. 1997;14:254–61. doi: 10.1007/BF02765826. http://dx.doi.org/10.1007/BF02765826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harton GL, Munné S, Surrey M, Grifo J, Kaplan B, McCulloh DH, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100:1695–703. doi: 10.1016/j.fertnstert.2013.07.2002. http://dx.doi.org/10.1016/j.fertnstert.2013.07.2002. [DOI] [PubMed] [Google Scholar]
- 28.Bopp BL, Alper MM, Thompson IE, Mortola J. Success rates with gamete intrafallopian transfer and in vitro fertilization in women of advanced maternal age. Fertil Steril. 1995;63:1278–83. doi: 10.1016/s0015-0282(16)57611-0. http://dx.doi.org/10.1016/S0015-0282. [DOI] [PubMed] [Google Scholar]
- 29.Spandorfer SD, Avrech OM, Colombero LT, Palermo GD, Rosenwaks Z. Effect of parental age on fertilization and pregnancy characteristics in couples treated by intracytoplasmic sperm injection. Hum Reprod. 1998;13:334–8. doi: 10.1093/humrep/13.2.334. http://dx.doi.org/10.1093/humrep/13.2.334. [DOI] [PubMed] [Google Scholar]
- 30.Padilla SL, Garcia JE. Effect of maternal age and number of in vitro fertilization procedures on pregnancy outcome. Fertil Steril. 1989;52:270–3. doi: 10.1016/s0015-0282(16)60854-3. http://dx.doi.org/10.1016/S0015-0282(16)60854-3. [DOI] [PubMed] [Google Scholar]
- 31.Gorelick AN, Karvir HV, Elashoff M, Parfitt DE, Copperman AB, Beim PY. Obesity has a greater impact on IVF success rates in patients with PCOS. Fertil Steril. 2014;102:e93. http://dx.doi.org/10.1016/j.fertnstert.2014.07.315. [Google Scholar]
- 32.Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R SART Writing Group. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod. 2011;26:245–52. doi: 10.1093/humrep/deq306. http://dx.doi.org/10.1093/humrep/deq306. [DOI] [PubMed] [Google Scholar]