Abstract
Objectives
Deep brain stimulation (DBS) is the surgical procedure for patients with advanced Parkinson’s disease. Globus pallidus internus (GPi) and subthalamic nucleus (STN) are the most targeted locations for the procedure. To investigate the variable efficiencies for the two different locations, we conducted a meta-analysis to compare both stimulation sites.
Materials and methods
A systematic search was performed in PubMed, Embase, and the Cochrane Library databases. Randomized controlled trials comparing the efficacies of GPi and STN DBS were included. Clinical outcomes of motor function, nonmotor function, and quality of life (QOL) were collected for the meta-analysis.
Results
Ten eligible trials with 1,034 patients were included in the analysis. Unified Parkinson’s disease rating scale III (UPDRS-III) scores were collected at 6, 12, and 24 months postsurgery separately to assess the motor function of the patients. A statistically significant effect in favor of the GPi DBS was obtained in the off-medication/on-stimulation phase of UPDRS-III at 12 months (mean difference [MD] =6.87, 95% confidence interval [95% CI]: 3.00–10.74, P=0.57, I2=0%). However, GPi DBS showed an opposite result at 24 months (MD =−2.46, 95% CI: −4.91 to −0.02, P=0.05, I2=0%). In the on-medication/on-stimulation phase, GPi DBS obtained a worse outcome compared with STN DBS (MD =−2.90, 95% CI: −5.71 to −0.09, P=0.05, I2=0%). Compared with STN DBS, increased dosage of levodopa equivalent doses was needed in GPi DBS (standardized MD =0.60, 95% CI: 0.46–0.74, P<0.00001, I2=24%). Meanwhile, Beck Depression Inventory II scores demonstrated that STN has a better performance (standardized MD =−0.31, 95% CI: −0.51 to −0.12, P=0.002, I2=0%). As for neurocognitive phase postsurgery, GPi DBS showed better performance in three of the nine tests, especially in verbal fluency. Use of GPi DBS was associated with a greater effect in eight of the nine subscales of QOL.
Conclusion
GPi and STN DBS significantly improve advanced Parkinson’s patients’ symptoms, functionality, and QOL. Variable therapeutic efficiencies were observed in both procedures, GPi and STN DBS. GPi DBS allowed greater recovery of verbal fluency and provided greater relief of depression symptoms. Better QOL was also obtained using GPi DBS. Meanwhile, GPi DBS was also associated with increased dosage of levodopa equivalent doses. The question regarding which target is superior remained open for discussion. An understanding of the target selection still depends on individual symptoms, neurocognitive/mood status, therapeutic goals of DBS (eg, levodopa reduction), and surgical expertise.
Keywords: advanced Parkinson’s disease, deep brain stimulation, globus pallidus internus, subthalamic nucleus
Introduction
Parkinson’s disease (PD) is a common, progressive, and age-related neurodegenerative disorder. It affects ~1% of people aged >60 years,1 with or without motor symptoms. Levodopa is the one of the main pharmaceutical treatments of PD at the early stage. However, for patients with advanced PD, long-term use of levodopa is usually associated with levodopa-induced dyskinesia and on–off fluctuations. In most cases, these complications cannot be fully controlled by medication therapies. Levodopa equivalent doses (LED) indicates the level of medication used both before and after deep brain stimulation (DBS). DBS is considered successful if postoperative levels are reduced compared to preoperative levels. DBS was first applied to PD patients in 1990s2 and was officially approved by the Food and Drug Administration in 2002. This technique has been used to treat PD in patients who do not respond adequately to levodopa treatment.3 With years of development in theory and practice, DBS became a proper choice for medication-refractory advanced PD.
Previous studies have shown that DBS was more effective in improving motor signs (eg, tremor, rigidity, bradykinesia) and quality of life (QOL) for advanced PD than pharmacologic treatment.4,5 The globus pallidus internus (GPi) and the subthalamic nucleus (STN) are the most common targets for DBS in PD patients. However, no standard protocol flowcharts are provided for neurosurgeons. Evidence from randomized controlled trials (RCTs) with various follow-up periods revealed inconsistent results,6–13 and previous meta-analyses mainly focused on the motor function compared between GPi and STN. Nonmotor symptoms, for example, depression and neurocognitive impairment, were excluded from the life quality evaluation system in PD patients.14 Moreover, three recent trials focusing on neurocognitive outcomes have been published and new evidence has been obtained.15–17 It is necessary to conduct a meta-analysis of the latest and fully covered evidence to assess the motor and nonmotor outcomes of both GPi DBS and STN DBS.
Materials and methods
Literature search
A systematic search was performed in PubMed, Embase, and the Cochrane Library databases from inception to December 30, 2015. We adopted exploded Medical Subject Headings (MeSH) terms and corresponding keywords in the electronic search process. The search terms used were (MeSH exp Parkinson Disease, and keywords Idiopathic Parkinson’s Disease, Lewy Body Parkinson’s Disease, Primary Parkinsonism), and (MeSH exp Deep Brain Stimulation and keywords Electrical Stimulation of the Brain and Deep Brain Stimulations), and (MeSH exp globus pallidus, and keywords Pallidum, Nucleus Subthalamicus), and (MeSH exp Subthalamic Nucleus, and keywords Luys Nucleus, Pallidums, Paleostriatum). No language or publication year restriction was imposed. We also manually checked the entire references of the selected studies to identify other potentially eligible studies that were not discovered during previous database searches.
Selection criteria
Two reviewers (ZGT and QZ) independently conducted the initial search. Retrieved literatures were imported into ENDNOTE (Thomson Reuters, Philadelphia, PA, USA), with duplication discarded. Unrelated literatures were excluded after careful scanning of titles and abstracts. Full-text articles of the remaining literatures were acquired to identify eligibility. Any discrepancy was resolved by discussion or decided by a third reviewer.
Published RCTs were included by meeting the following criteria: 1) population: patients with advanced PD were responsive to levodopa; 2) intervention: GPi DBS or STN DBS (either bilateral or unilateral); 3) comparison: STN DBS or GPi DBS (either bilateral or unilateral); 4) one or more of the following outcomes: scores of unified PD rating scale III (UPDRS-III), scores of Beck Depression Inventory II (BDI-II), LED, neurocognitive status, and QOL. Literatures were excluded for the following reasons: 1) subjects in each team were <10; 2) maximum follow-up time was <6 months; and (3) data from conference abstracts or literatures that could not be extracted.
Data collection and risk of bias assessment
Two authors (ZGT and QZ) performed data extraction from the included studies. Also they completed the risk of bias assessment independently. The data collected contained the following information: the first author, year of publication, country, subject size, surgical modus, assessment time points, and characteristics of the patients. Extracted data were input into an excel file. Risk of bias was assessed with the risk-of-bias tool provided by Cochrane Collaboration.18 Each study was assessed for the following six items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Discussion or a third author resolved discrepancies.
Statistical analysis
All the outcomes were displayed in consistent data. Mean differences (MDs) with 95% confidence intervals (95% CIs) were calculated for UPDRS-III, since they were pooled in different subgroups of follow-up time. Standardized mean differences (SMDs) with 95% CIs were reported for the rest of the outcomes, since they were collected at the maximum follow-up time points or were collected in different data types (changes from baseline data or end point data). P-value <0.05 was considered statistically significant. The heterogeneity across studies was calculated using χ2 and I2 tests. Once the heterogeneity was small (I2≤50%), the fixed-effects model was used; otherwise, the random effects model was used. Meta-analysis was performed by means of the RevMan 5.3 (The Cochrane Collaboration, London, UK).
Results
Trial selection
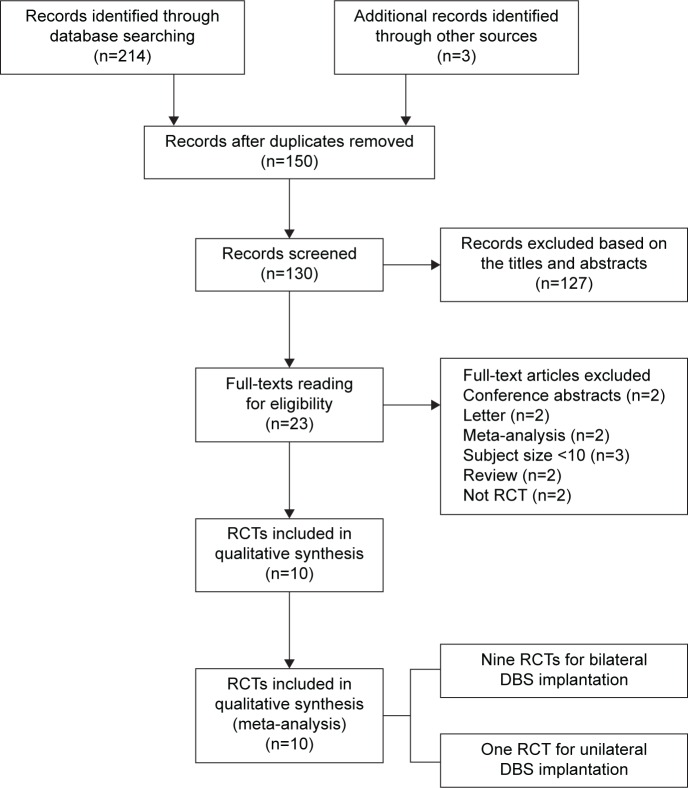
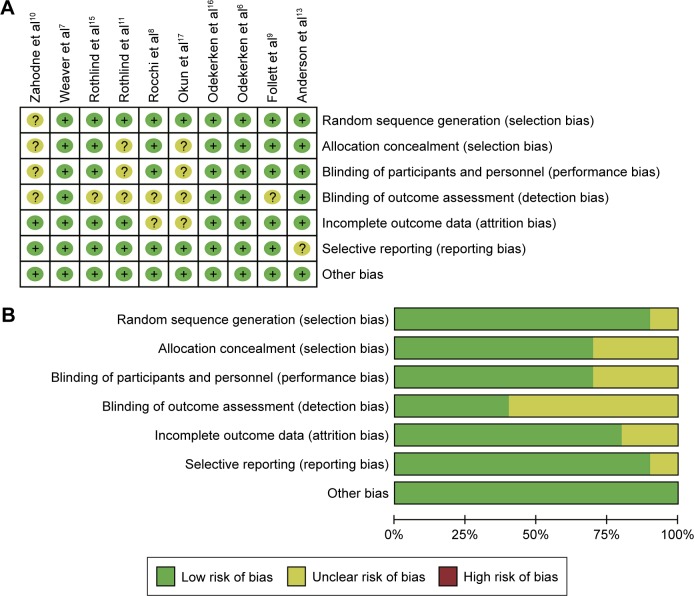
The PRISMA statement flow diagram displayed the process of literature search and selection, as shown in Figure 1. Initially, 217 records were collected. By excluding duplicated articles and screening titles, 23 potential eligible articles remained. By intensive reading, 13 undesired articles were excluded. Ten RCTs were included in our meta-analysis at the end. All characteristics of the included RCTs are summarized in Table 1. Patients in nine trials underwent bilateral GPi or STN implantation, and patients in one trial underwent unilateral implantation. The results of risk of bias assessment are summarized in Figure 2A and B.
Figure 1.
Flow diagram of selection of RCTs.
Abbreviations: RCTs, randomized controlled trials; DBS, deep brain stimulation.
Table 1.
Characteristics of included randomized controlled trials comparing GPi DBS with STN DBS for advanced Parkinson’s disease
| Study | Country | Target | Surgical modus | Subject size, n | Male/female, n | Age (years), mean ± SD | Disease duration (years), mean ± SD | Outcome measure | Assessment time points |
|---|---|---|---|---|---|---|---|---|---|
| Odekerken et al16 | The Netherlands | GPi | Bilateral | 58 | 40/18 | 59.2±7.7 | 10.9±4.0 | Neurocognitive status | Baseline, 12 months |
| STN | 56 | 42/14 | 60.3±7.4 | 12.3±5.5 | |||||
| Rothlind et al15 | USA | GPi | Bilateral | 80 | NA | 61.3±8.9 | 11.0±4.7 | Neurocognitive status | Baseline, 6 months, 12 months, 24 months |
| STN | 64 | 61.3±8.5 | 11.0±5.0 | ||||||
| Okun et al17 | USA | GPi | Bilateral | 14 | 6/8 | 60.1±5.5 | 11.5±3.3 | UPDRS-III, LED, BDI-II | Baseline, 2 months, 4 months, 6 months, 12 months |
| STN | 16 | 3/13 | 58.0±10.7 | 12.1±4.5 | |||||
| Odekerken et al6 | The Netherlands | GPi | Bilateral | 65 | 44/21 | 59.1±7.8 | 10.8±4.2 | UPDRS-III, LED | Baseline, 12 months |
| STN | 63 | 44/19 | 60.9±7.6 | 12.0±5.3 | |||||
| Weaver et al7 | USA | GPi | Bilateral | 89 | 77/12 | 60.4±8.3 | 11.4±4.9 | UPDRS-III, LED, PDQ-39 | Baseline, 3 months, 6 months, 12 months, 18 months, 24 months, 36 months |
| STN | 70 | 56/14 | 60.7±8.9 | 11.3±4.7 | |||||
| Rocchi et al8 | Italy and USA | GPi | Bilateral | 14 | 13/1 | 61.1±8.4 | 12.9±10.7 | UPDRS-III, LED | Baseline, 6 months |
| STN | 15 | 11/4 | 61.4±5.5 | 11.9±4.8 | |||||
| Follett et al9 | USA | GPi | Bilateral | 152 | 133/19 | 61.8±8.7 | NA | UPDRS-III, LED, BDI-II | Baseline, 6 months, 24 months |
| STN | 174 | 116/31 | 61.9±8.7 | Neurocognitive status, PDQ-39 | |||||
| Zahodne et al10 | USA | GPi | Unilateral | 22 | 16/6 | 61.3±5.5 | 12.4±3.6 | UPDRS-III, LED, BDI-II | Baseline, 6 months |
| STN | 20 | 14/6 | 61.3±9.0 | 13.6±3.9 | PDQ-39 | ||||
| Rothlind et al11 | USA | GPi | Bilateral | 23 | 18/5 | 60.2±8.83 | 13.3±6.4 | UPDRS-III, BDI-II, LED | Baseline, 6 months, 21 months |
| STN | 19 | 15/4 | 61.4±10.11 | 12.9±4.3 | Neurocognitive status | ||||
| Anderson et al13 | Switzerland | GPi | Bilateral | 10 | NA | 54±12 | 10.3±2 | UPDRS-III | Baseline, 12 months |
| STN | 10 | 61±9 | 15.6±5 |
Abbreviations: GPi, globus pallidus interna; STN, subthalamic nucleus; DBS, deep brain stimulation; NA, not available; UPDRS-III, unified Parkinson’s disease rating scale III; LED, levodopa equivalent doses; BDI-II, Beck Depression Inventory II; PDQ-39, Parkinson’s Disease Questionnaire-39; SD, standard deviation.
Figure 2.
Risk of bias graph.
Notes: (A) Each risk of bias item for each included study. (B) The overall risk of bias is relatively low. “+” indicates yes; “?” indicates not clear.
UPDRS-III (motor)
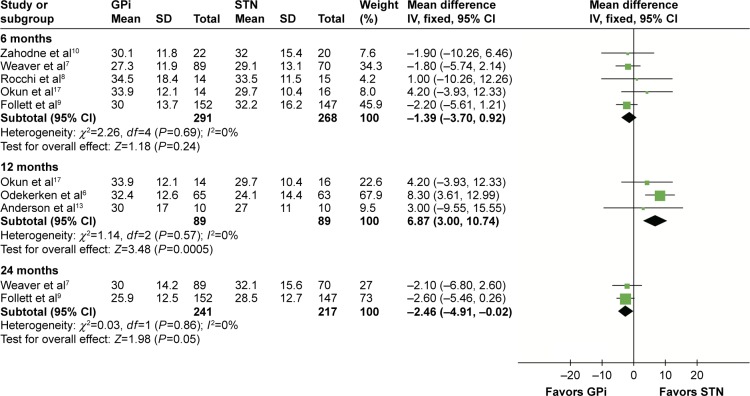
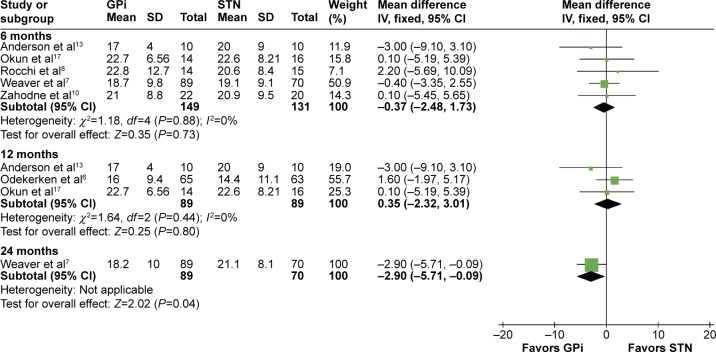
According to different follow-up periods, subgroup analyses including postsurgery 6, 12, and 24 months were conducted. In the off-medication/on-stimulation phase, no significant difference was found between GPi DBS and STN DBS at 6 months (MD =−1.39, 95% CI: −3.70 to 0.92, P=0.24, I2=0%). At 12 months, a statistically significant effect in favor of the GPi DBS was obtained in the off-medication/on-stimulation phase of UPDRS-III (MD =6.87, 95% CI: 3.00–10.74, P=0.57, I2=0%). However, GPi DBS showed an opposite result in 24 months (MD =−2.46, 95% CI: −4.91 to −0.02, P=0.05, I2=0%). Details are shown in Figure 3. In the on-medication/on-stimulation phase, the study reduced to a nonsignificant level when GPi DBS was compared with STN DBS at 6 months (MD =−0.37, 95% CI: −2.48 to 1.73, P=0.73, I2=0%) and 12 months (MD =0.35, 95% CI: −2.32 to 3.01, P=0.80, I2=0%). However at 24 months, GPi DBS obtained a worse outcome compared with STN DBS (MD =−2.90, 95% CI: −5.71 to −0.09, P=0.05, I2=0%; Figure 4).
Figure 3.
Forest plot of mean difference of UPDRS-III score in the off-medication/on-stimulation state, stratified by follow-up length.
Abbreviations: UPDRS-III, unified Parkinson’s disease rating scale III; GPi, globus pallidus interna; STN, subthalamic nucleus; IV, inverse variance; CI, confidence interval.
Figure 4.
Forest plot of mean difference of UPDRS-III score in the on-medication/on-stimulation state, stratified by follow-up length.
Abbreviations: UPDRS-III, unified Parkinson’s disease rating scale III; GPi, globus pallidus interna; STN, subthalamic nucleus; IV, inverse variance; CI, confidence interval.
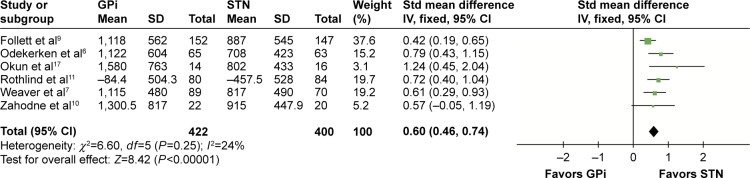
Levodopa equivalent doses
Six studies that recruited 822 patients were provided with levodopa equivalent study (LED). Compared with STN DBS, increased LED was needed in GPi DBS (SMD =0.60, 95% CI: 0.46–0.74, P<0.00001, I2=24%; Figure 5).
Figure 5.
Forest plot of standardized mean difference of levodopa equivalent doses.
Abbreviations: GPi, globus pallidus interna; STN, subthalamic nucleus; IV, inverse variance; CI, confidence interval; Std, standardized.
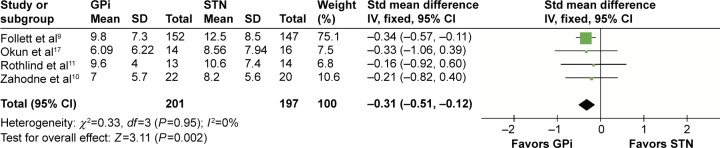
Beck Depression Inventory II
Four studies that involved 398 patients were provided with BDI-II. BDI-II scores demonstrated that STN has a better performance (SMD =−0.31, 95% CI: −0.51 to −0.12, P=0.002, I2=0%; Figure 6).
Figure 6.
Forest plot of standardized mean difference of Beck Depression Inventory II.
Abbreviations: GPi, globus pallidus interna; STN, subthalamic nucleus; IV, inverse variance; CI, confidence interval; Std, standardized.
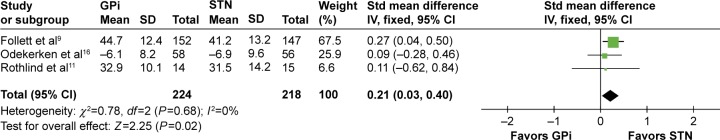
Neurocognitive status
Various subscales were used to evaluate the neurocognitive status with DBS implantation, including, but not limited to, verbal fluency, processing speed, language, executive function, and dementia status. Each subscale had different methods to quantify the evaluation. Three of the nine tests scores showed statistical significance between GPi and STN DBS. The significant differences are in favor of GPi DBS in semantic fluency test (SMD =0.21, 95% CI: 0.03–0.40, P=0.02, I2=0%; Figure 7), phonemic fluency test (SMD =0.19, 95% CI: 0.00–0.38, P=0.05, I2=0%), and stroop word reading test (SMD =0.33, 95% CI: 0.10–0.56, P=0.005, I2=41%). No statistical significance was observed in other tests, including stroop color naming test (SMD =0.19, 95% CI: −0.29 to 0.27, P=0.44, I2=42%), trail making test A (SMD =0.05, 95% CI: −0.18 to 0.27, P=0.67, I2=27%), Boston naming test (SMD =0.11, 95% CI: −0.05 to 0.27, P=0.16, I2=0%), trail making test B (SMD =0.10, 95% CI: −0.27 to 0.47, P=0.58, I2=41%), stroop color–word interference test (SMD =0.14, 95% CI: −0.09 to 0.37, P=0.23, I2=0%), and Mattis dementia rating scale (SMD =0.14, 95% CI: −0.05 to 0.33, P=0.15, I2=0%). Comparison items are displayed in Table 2.
Figure 7.
Forest plot of standardized mean difference of semantic fluency test.
Abbreviations: GPi, globus pallidus interna; STN, subthalamic nucleus; IV, inverse variance; CI, confidence interval; Std, standardized.
Table 2.
Entire results of neurocognitive status comparison between GPi DBS and STN DBS
| Measure | Included study number | SMD, IV, fixed, 95% CI | Test for heterogeneity
|
Test for overall effect
|
||
|---|---|---|---|---|---|---|
| I2 (%) | P-value | Z | P-value | |||
| Verbal fluency | ||||||
| Semantic fluency | 3 | 0.21 (0.03 to 0.40) | 0 | 0.68 | 2.25 | 0.02 |
| Phonemic fluency | 3 | 0.19 (0.00 to 0.38) | 0 | 0.79 | 1.97 | 0.05 |
| Processing speed | ||||||
| Stroop word reading | 3 | 0.33 (0.10 to 0.56) | 41 | 0.16 | 2.83 | 0.005 |
| Stroop color naming | 3 | 0.19 (−0.29 to 0.67) | 42 | 0.15 | 0.78 | 0.44 |
| Trail making test A | 3 | 0.05 (−0.18 to 0.27) | 27 | 0.26 | 0.39 | 0.69 |
| Language | ||||||
| Boston naming test | 3 | 0.11 (−0.05 to 0.27) | 0 | 0.86 | 1.41 | 0.16 |
| Executive function | ||||||
| Trail making test B | 3 | 0.10 (−0.27 to 0.47) | 41 | 0.16 | 0.55 | 0.58 |
| Stroop color–word interference | 3 | 0.14 (−0.09 to 0.37) | 0 | 0.84 | 1.21 | 0.23 |
| Dementia | ||||||
| MDRS | 2 | 0.14 (−0.05 to 0.33) | 0 | 0.95 | 1.44 | 0.15 |
Abbreviations: GPi, globus pallidus interna; STN, subthalamic nucleus; DBS, deep brain stimulation; SMD, standardized mean difference; IV, inverse variance; CI, confidence interval; MDRS, Mattis dementia rating scale.
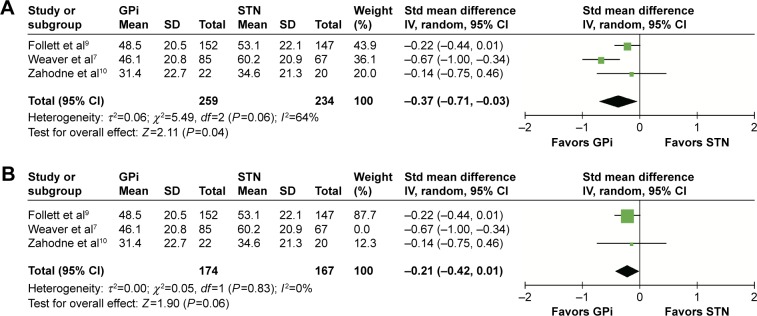
Quality of life
QOL was considered to be the most important outcome since it reflected comprehensive effects of DBS regarding motor and nonmotor outcomes.19 Three studies that recruited nearly 500 patients were provided with PDQ-39, which was widely used to assess the health-related QOL with PD patients. Eight of the nine subscales showed a statistical significance between the two targets. Compared with STN DBS, GPi DBS had a better function in mobility (SMD =−0.35, 95% CI: −0.53 to −0.17, P=0.0001, I2=0%), activities of daily living (SMD =−0.50, 95% CI: −0.68 to −0.32, P<0.00001, I2=0%), emotional well-being (SMD =−0.26, 95% CI: −0.44 to −0.09, P=0.004, I2=0%), social support (SMD =−0.21, 95% CI: −0.39 to −0.04, P=0.02, I2=0%), cognition (SMD =−0.26, 95% CI: −0.43 to −0.08, P=0.004, I2=0%), communication (SMD =−0.37, 95% CI: −0.71 to −0.03, P=0.04, I2=64%), body discomfort (SMD =−0.21, 95% CI: −0.39 to −0.04, P=0.02, I2=0%), and single index (SMD =−0.33, 95% CI: −0.51 to −0.15, P=0.0003, I2=0%). As for stigma, GPi DBS had the tendency of better function; however, the difference was not significant (SMD =−0.13, 95% CI: −0.30 to −0.05, P=0.16, I2=0%). The heterogeneity was substantial (I2=64%, P=0.06; Figure 8A) regarding the subscale “communication”, and random-effects model was used. Sensitivity analysis was performed to determine the reason. The heterogeneity was reduced greatly (I2=0%, P=0.83) when the study by Weaver et al7 was excluded. Data from this trial were collected 36 months after DBS implantation, which was much longer than the other two. This could contribute heterogeneity to a larger extent. By excluding the “Weaver et al7” study, GPi DBS had a tendency of better function; however, the difference was no longer significant (SMD =−0.21, 95% CI: −0.42 to 0.01, P=0.06; Figure 8B). The results of QOL are summarized in Table 3.
Figure 8.
Forest plot of standardized mean difference of the subscale “communication”.
Notes: (A) GPi had lower score and the difference was significant. But the heterogeneity between studies was substantial. (B) The heterogeneity reduced greatly (I2=0%, P=0.06) when the study “Weaver et al7” was excluded.
Abbreviations: GPi, globus pallidus interna; STN, subthalamic nucleus; IV, inverse variance; CI, confidence interval; Std, standardized.
Table 3.
Entire results of quality of life comparison between the two targets
| PDQ-39 | SMD, IV, fixed, 95% CI | Test for heterogeneity
|
Test for overall effect
|
||
|---|---|---|---|---|---|
| I2 (%) | P-value | Z | P-value | ||
| Mobility | −0.35 (−0.53 to −0.17) | 0 | 0.74 | 3.88 | 0.0001 |
| Activities of daily living | −0.50 (−0.68 to −0.32) | 0 | 0.57 | 5.44 | <0.00001 |
| Emotional well-being | −0.26 (−0.44 to −0.09) | 0 | 0.38 | 2.90 | 0.004 |
| Stigma | −0.13 (−0.30 to 0.05) | 0 | 0.89 | 1.39 | 0.16 |
| Social support | −0.21 (−0.39 to −0.04) | 0 | 0.65 | 2.34 | 0.02 |
| Cognition | −0.26 (−0.43 to −0.08) | 0 | 0.95 | 2.85 | 0.004 |
| Communication | −0.37 (−0.71 to −0.03)a | 64 | 0.06 | 2.11 | 0.04 |
| Bodily discomfort | −0.21 (−0.39 to −0.04) | 0 | 0.46 | 2.38 | 0.02 |
| Single index | −0.33 (−0.51 to −0.15) | 0 | 0.91 | 3.63 | 0.0003 |
Note:
The random-effects model was used because of significant heterogeneity (I2=64%, P=0.06).
Abbreviations: SMD, standardized mean difference; IV, inverse variance; CI, confidence interval.
Discussion
Main findings and implications for clinical practice
Motor control is the main therapeutic goal of advanced PD patients who undergo DBS. UPDRS-III (motor subscale) is widely used to assess PD patients’ motor function. We analyzed the UPDRS-III scores at 6, 12, and 24 months follow-up points postsurgery. In the off-medication/on-stimulation phase, significant difference was obtained at 12 and 24 months. GPi DBS was 6.87 points higher than STN DBS at 12 months. However, GPi DBS was 2.46 points lower than STN DBS at 24 months, and this difference may have little clinical significance since 5 points is a minimal scale of clinically important change.20 In the on-medication/on-stimulation state, significant difference was seen at 24 months, with GPi DBS 2.90 points lower than STN DBS. This difference may have little clinical significance too. Generally speaking, the improvement of motor function is comparable between the two targets, though scores of UPDRS-III may have a few differences. Equivalent efficacy in motor function of the two targets means neurosurgeon should pay more attention to nonmotor outcome when deciding the suitable target for patients.
The levodopa equivalent doses decreased more in patients with implanted STN DBS. This advantage may be an important premise of treatment for PD patients who suffer from levodopa-induced dyskinesia, even for those who have poor compliance with long-term high doses of levodopa intake. However, neurosurgeons have to avoid aggressive medication reduction after STN DBS, since apathy and depressive symptoms may occur once levodopa was rapidly withdrawn.21
Nonmotor symptoms, including mood disorder and neurocognitive impairment, are important manifestations of PD, which may exacerbate PD process and bring down patients’ QOL. Previous studies reported negative impact of DBS on mood and neurocognition, especially for STN DBS. However, their outcomes had substantial heterogeneity and controversial results.9,10 The depression level was measured with BDI-II. Our meta-analysis revealed the overall BDI-II scores after implantation. STN DBS was more favorable in this evaluation. A possible interpretation was that STN DBS may easily affect nearby fiber pathways regulating limbic functions.11 Therefore, for PD patients with depression symptoms, GPi may be the preferred target. On the other hand, frequent mood tests are necessary for patients undergoing STN DBS during postoperative follow-up. As for the neurocognition, STN DBS showed worse performance in the subscale of verbal fluency, including both semantic fluency and phonemic fluency. Okun et al22 thought that this was a surgical rather than a stimulation-induced effect because verbal fluency still deteriorated in the off-STN DBS state. Jahanshahi et al23 have recently found that STN plays a role in inhibitory and executive control, including cognitive domain. Electrode implantation in STN might bring deficits in inhibitory control. Moreover, GPi DBS performed better in the stroop word reading test, although there was no significance between the targets in the other two tests with regard to the subscale processing speed. No significance was found between the targets in the other subscales of neurocognition.
QOL reflects the overall outcome of DBS implantation. GPi DBS shows greater improvement in QOL. The importance of the superiority of GPi DBS should be interpreted critically since only three studies were provided with PDQ-39, plus one of the studies had a few differences of PDQ-39 scores at baseline.7
Strengths and limitations
Our meta-analysis was the latest and the most comprehensive one, including ten RCTs and 1,034 cases. A major strength of this meta-analysis was comparing the neurocognitive function and QOL between GPi DBS and STN DBS. The importance of the two indexes may be underestimated before and remained heterogeneous in different studies.
Our meta-analysis also had the following limitations: 1) the studies involved were conducted with various implantation techniques, stimulators, stimulation parameters, and postoperative management. Therefore, potential risks of significant heterogeneity were undefined; 2) analysis was mainly based on data from bilateral implantations. However, the trial with 42 patients who underwent unilateral implantation was also included.10 The results for unilateral implantation may be inconclusive, since unilateral implantation can have bilateral implantation benefits;24 3) some adopted data were collected at 6 months after surgery, and this may be too early to check the efficacy.
Conclusion
Globus pallidus stimulation and STN stimulation significantly improve advanced Parkinson’s patients’ symptoms, functionality, and QOL. Variable therapeutic efficiencies were observed in both procedures, GPi and STN DBS. GPi DBS allowed greater recovery of verbal fluency and provided greater relief of depression symptoms. Better QOL was obtained using GPi DBS. Meanwhile, GPi DBS was also associated with higher levodopa equivalent doses. The question regarding which target is superior remained open for discussion. An understanding of the target selection still depends on individual symptoms, neurocognitive/mood status, therapeutic goals of DBS (eg, levodopa reduction), and surgical expertise.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurology. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 1998;339(16):1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 3.Okun MS. Deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2012;367(16):1529–1538. doi: 10.1056/NEJMct1208070. [DOI] [PubMed] [Google Scholar]
- 4.Williams A, Gill S, Varma T, et al. PD SURG Collaborative Group Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 2010;9(6):581–591. doi: 10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver FM, Follett K, Stern M, et al. CSP 468 Study Group Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odekerken VJ, van Laar T, Staal MJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013;12(1):37–44. doi: 10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- 7.Weaver FM, Follett KA, Stern M, et al. CSP 468 Study Group Randomized trial of deep brain stimulation for Parkinson disease: thirty-six-month outcomes. Neurology. 2012;79(1):55–65. doi: 10.1212/WNL.0b013e31825dcdc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocchi L, Carlson-Kuhta P, Chiari L, Burchiel KJ, Hogarth P, Horak FB. Effects of deep brain stimulation in the subthalamic nucleus or globus pallidus internus on step initiation in Parkinson disease: laboratory investigation. J Neurosurg. 2012;117(6):1141–1149. doi: 10.3171/2012.8.JNS112006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Follett KA, Weaver FM, Stern M, et al. CSP 468 Study Group Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362(22):2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 10.Zahodne LB, Okun MS, Foote KD, et al. Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus as compared to the subthalamic nucleus. J Neurol. 2009;256(8):1321–1329. doi: 10.1007/s00415-009-5121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothlind JC, Cockshott RW, Starr PA, Marks WJ., Jr Neuropsychological performance following staged bilateral pallidal or subthalamic nucleus deep brain stimulation for Parkinson’s disease. J Int Neuropsychol Soc. 2007;13(1):68–79. doi: 10.1017/S1355617707070105. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Christine CW, Starr PA, Marks WJ., Jr Effects of unilateral subthalamic and pallidal deep brain stimulation on fine motor functions in Parkinson’s disease. Mov Disord. 2007;22(5):619–626. doi: 10.1002/mds.21300. [DOI] [PubMed] [Google Scholar]
- 13.Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62(4):554–560. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- 14.Prakash KM, Nadkarni NV, Lye WK, Yong MH, Tan EK. The impact of non-motor symptoms on the quality of life of Parkinson’s disease patients: a longitudinal study. Eur J Neurol. 2016;23(5):854–860. doi: 10.1111/ene.12950. [DOI] [PubMed] [Google Scholar]
- 15.Rothlind JC, York MK, Carlson K, et al. CSP-468 Study Group Neuropsychological changes following deep brain stimulation surgery for Parkinson’s disease: comparisons of treatment at pallidal and subthalamic targets versus best medical therapy. J Neurol Neurosurg Psychiatry. 2015;86(6):622–629. doi: 10.1136/jnnp-2014-308119. [DOI] [PubMed] [Google Scholar]
- 16.Odekerken VJ, Boel JA, Geurtsen GJ, et al. NSTAPS Study Group Neuropsychological outcome after deep brain stimulation for Parkinson disease. Neurology. 2015;84(13):1355–1361. doi: 10.1212/WNL.0000000000001419. [DOI] [PubMed] [Google Scholar]
- 17.Okun MS, Wu SS, Fayad S, et al. Acute and chronic mood and apathy outcomes from a randomized study of unilateral STN and GPi DBS. PLoS One. 2014;9(12):e114140. doi: 10.1371/journal.pone.0114140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gotzsche PC, et al. Cochrane Bias Methods Group. Cochrane Statistical Methods Group The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sako W, Miyazaki Y, Izumi Y, Kaji R. Which target is best for patients with Parkinson’s disease? A meta-analysis of pallidal and subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2014;85(9):982–986. doi: 10.1136/jnnp-2013-306090. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR, NMSS Validation Group The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399–406. doi: 10.1002/mds.23462. [DOI] [PubMed] [Google Scholar]
- 21.Thobois S, Lhommee E, Klinger H, et al. Parkinsonian apathy responds to dopaminergic stimulation of D2/D3 receptors with piribedil. Brain. 2013;136(pt 5):1568–1577. doi: 10.1093/brain/awt067. [DOI] [PubMed] [Google Scholar]
- 22.Okun MS, Fernandez HH, Wu SS, et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009;65(5):586–595. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahanshahi M, Obeso I, Baunez C, Alegre M, Krack P. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128–140. doi: 10.1002/mds.26049. [DOI] [PubMed] [Google Scholar]
- 24.Taba HA, Wu SS, Foote KD, et al. A closer look at unilateral versus bilateral deep brain stimulation: results of the National Institutes of Health COMPARE cohort. J Neurosurg. 2010;113(6):1224–1229. doi: 10.3171/2010.8.JNS10312. [DOI] [PubMed] [Google Scholar]