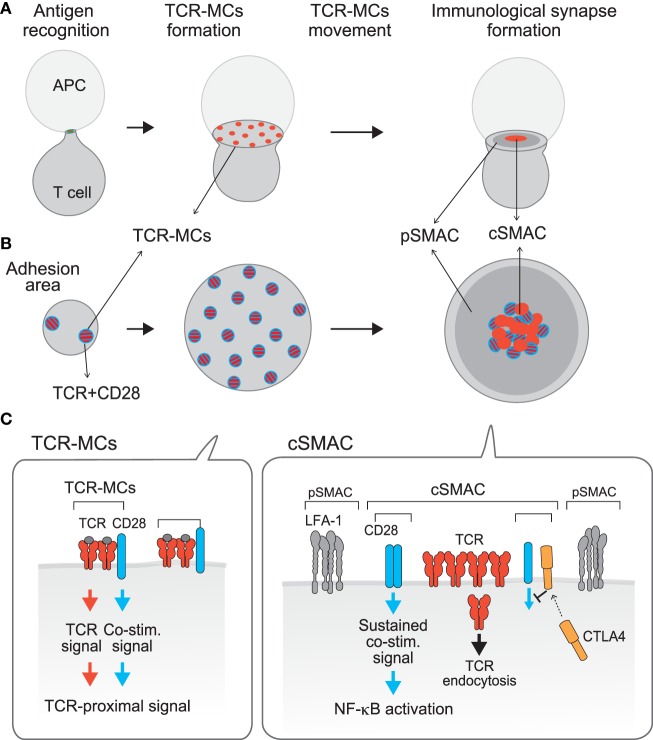
Figure 1.
Compartmentalization of TCR activation signals: TCR–microcluster and cSMAC. [(A), x–y axis; (B), z-axis; (C), a model for the assembly on the membrane] Upon Ag recognition, T cells form a conjugate with the APC (left) and generate TCR–microclusters (MCs) at the interface with the APC. TCR–MCs contain TCRs (red) and the proximal signaling molecules as well as the CD28 co-stimulation receptor (blue), and induce the initial activation signal (middle). After maximum spreading, TCR–MCs begin to move toward the center of the interface to form the cSMAC (right). It was noted that there is a CD3hi region (red) and a CD3lo region (mixture of red and blue) within the cSMAC; the CD3hi region is rigid and may represent the site for TCR endocytosis, whereas the CD3lo region is dynamically regulated and various costimulation molecules as CD28 and CTLA-4 are co-localized. Thus, we named this CD3lo region the “signaling cSMAC.” In the cSMAC, the TCR complex is subjected to endocytosis/degradation for negative regulation, whereas CD28 recruits PKCθ and CARMA1 to induce a sustained co-stimulation signal leading to downstream events such as of NF-κB activation. The inhibitory co-stimulation receptor CTLA4 is translocated to the same cSMAC area as CD28 and competes with CD28 to eject CD28-PKCθ from the cSMAC, resulting in inhibition of activation. Thus, the TCR activation signal is regulated by spatially distinct signals: The Ag recognition signal as “Signal 1” is mediated by TCR–MCs and a sustained co-stimulation signal as “Signal 2” is mediated by the signaling region of the cSMAC.