| Oil-in-water (o/w) emulsion |
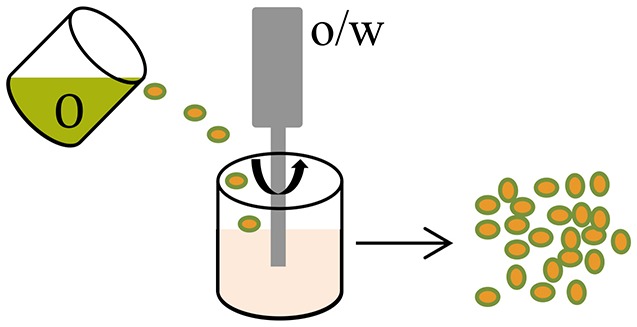 |
|
Low encapsulation efficiency especially for water-soluble payloads Solvent residuals Low yield, agglomeration of sticky particles
|
Varde and Pack, 2004
|
| Water-in-oil-in-water (w/o/w) emulsion |
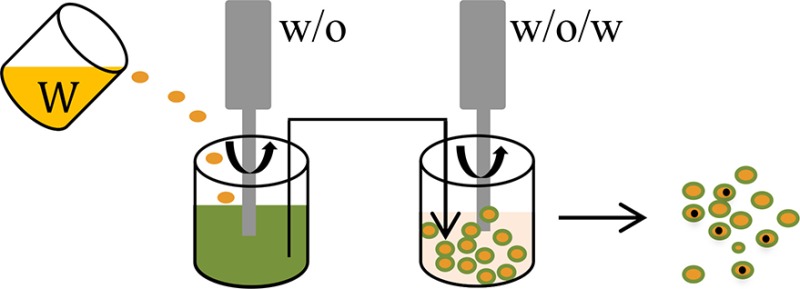 |
|
|
|
| Supercritical CO2 (scCO2) |
 |
|
|
Falco et al., 2012; Dhanda et al., 2013
|
| Spray drying |
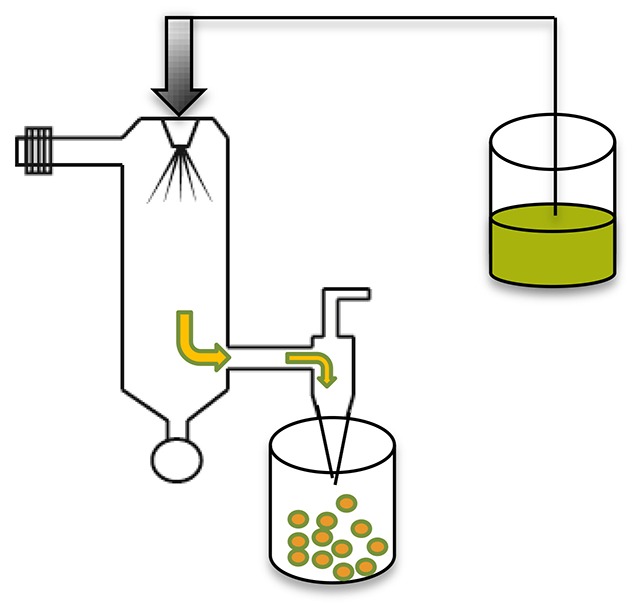 |
Can encapsulate wide range of drugs/peptides/proteins into microparticles without significant loss Final drying step not required One step and reproducible Atomizers (nozzles) eliminate the need for complicated pre-preparation processes and enable continuous manufacture by utilization of liquid feeds via two separate channels
|
Adhesion of microparticles to inner walls of the spray-dryer Not suitable for temperature-sensitive compounds Difficult to control particle size Low yield, agglomeration of sticky particles
|
Makadia and Siegel, 2011; Sosnik and Seremeta, 2015; Wan and Yang, 2016
|
| CES (Other modification, such as, coaxial tri-capillary electrospray, Emulsion-coaxial electrospinning) |
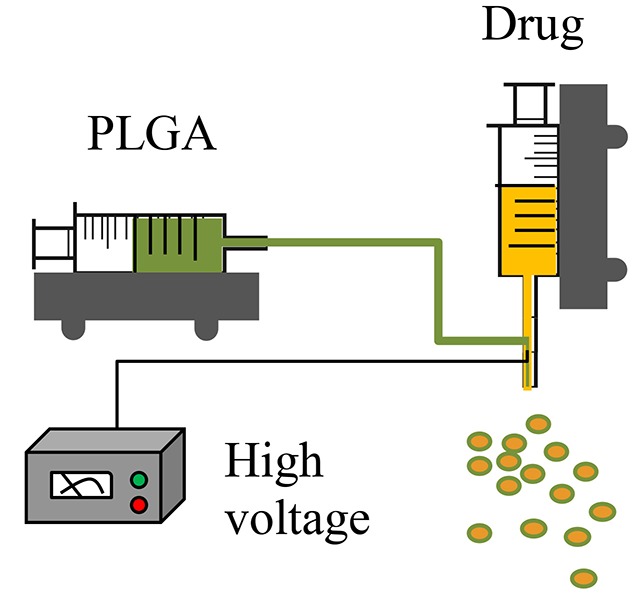 |
Nearly 100% encapsulation rate Useful for encapsulating water-soluble molecules Protects biologically active payloads from processing-induced damage Potential to control particle morphology with flexibility and reproducibility for both micro- and nanoparticle size ranges
|
At early stage; requires further development Standardized protocols and systematic process controls not available as yet Lack of an effective particle collection method; commonly used one-step collection methods cannot facilitate shell hardening, or maintain particle morphology or prevent particle aggregation Lack of a more productive nozzle design
|
Lee et al., 2011; Viry et al., 2012; Zhang et al., 2012; Zamani et al., 2014; Yuan et al., 2015
|
| Microfluidics (Other modification, such as, capillary microfluidics coupled with solvent evaporation) |
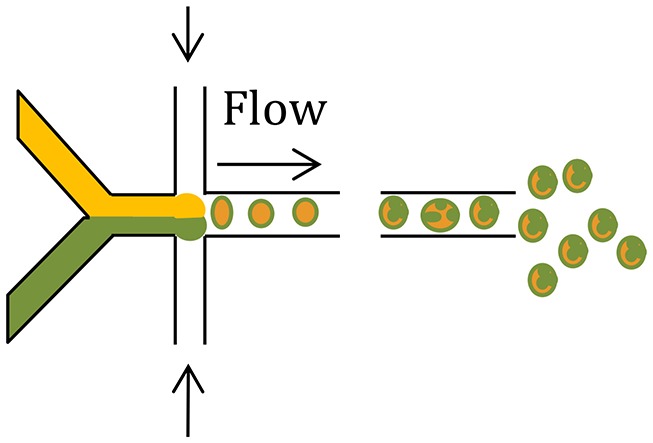 |
Ultra-small quantities of reagents needed Precise control over drug release rate, drug loading efficiency, particle shell thickness, particle shape and size Multiple components are easily generated using single-step emulsification
|
|
Demello, 2006; Hung et al., 2010; Xie et al., 2012; Cho and Yoo, 2015; Leon et al., 2015
|
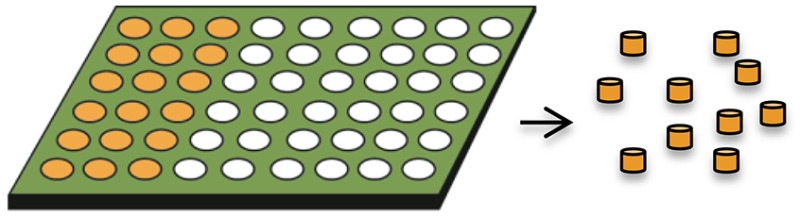
| Hydrogel template |
 |
|
|
Acharya et al., 2010a,b; Malavia et al., 2015
|







