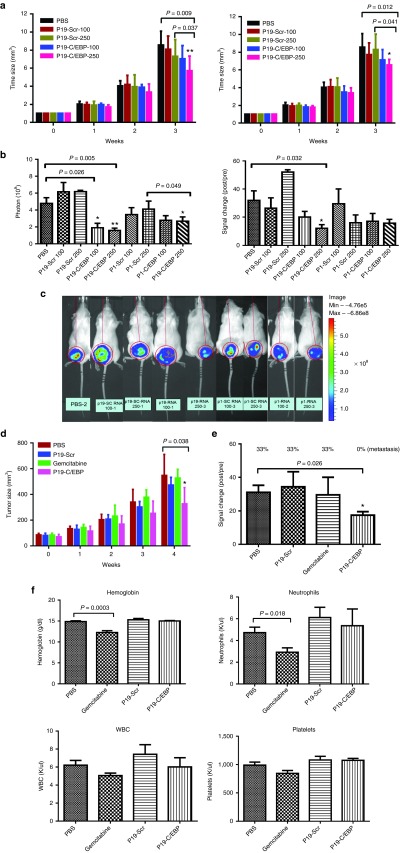
Figure 4.
In vivo effects of P19 and P1- C/EBPα-saRNA. (a) P19- and P1-conjugated C/EBPα-saRNA aptamers were injected in PANC-1 engrafted mice via tail vein injection at 100 and 250 pmol. Tumor size was calculated by the formula 0.52 × length × width × width. Data are presented as the mean ± SD (n = 4 each group) (b) Tumor growth was monitored by evaluating bioluminescence before the first injection and 1 week after the last injection. The photons (left) and bioluminescent signal changes (right) were quantified. (c) Bioluminescent images of the xenografts are a representative of each treatment groups. (d) Comparison of the antitumor effect of C/EBPα-saRNA with gemcitabine. P19-conjugated C/EBPα-saRNA aptamers were injected in PANC-1 engrafted mice by tail vein injection at 1 nmol. Gemcitabine were injected by i.p. at 3 mg on day 5 and 7 for 4 weeks. Data are presented as the mean ± SD (n = 6 each group) (e) The antitumor effects in gemcitabine-resistant AsPC-1 cells in vivo were assessed. P19-conjugated C/EBPα-saRNA aptamers were injected in AsPC-1-engrafted mice by tail vein injection at 1 nmol. Data are presented as the mean ± SD (PBS: n = 6, P19-CEBP: n = 5, P19-Scr: n = 3, gemcitabine: n = 3). Tumor growth was monitored by evaluating bioluminescence before the first injection and 1 week after the last injection. The percentage of the cells that had metastasized to the ascites was measured. (f) To assess cytotoxicity following gemcitabine treatment, the blood parameter for hemoglobin, white blood cell count, platelets, and neutrophils were measured. Data are presented as the mean ± SD (n = 6 each group).