Abstract
Dysphagia is an alarm symptom requiring a prompt investigation. Different benign and malignant diseases may present such a symptom. We describe a case of a 79-year-old patient who complained of fluctuating dysphagia episodes following solid food ingestion in the previous 5 months with mild weight loss. No other gastrointestinal symptoms were present. The patient was referred by the General Practitioner for a videofluoroscopic swallow examination which revealed nodularity of mucosa surface in the oropharynx, esophagus, fundus, and gastric body. Upper endoscopy confirmed the feature, also showing a normal mucosa of the antrum and duodenum. The histological examination revealed a mantle cell lymphoma (MCL). A stage III, MCL involving the esophagus and proximal stomach was eventually diagnosed. Esophageal MCL localization is extremely rare, and this is the first report showing a clinical onset with dysphagia.
Keywords: Mantle cell lymphoma, dysphagia, esophagus, stomach, endoscopy
Introduction
Dysphagia is considered an alarm symptom deserving a prompt investigation. Such a symptom may occur in different benign and malignant diseases, including reflux esophagitis, eosinophilic esophagitis, achalasia, as well as esophageal and cardia cancer [1,2]. Lymphomas may involve the gastrointestinal (GI) tract, but the esophageal localization is extremely rare. We report a case of primary mantle cell lymphoma (MCL) involving the esophagus and stomach presenting with dysphagia.
Case report
A 79-year-old man was referred by his General Practitioner to our Radiology Unit in the August 2015 to perform a swallow study. He complained of fluctuating dysphagia episodes following solid food ingestion over the last 5 months, with mild weight loss. No other GI symptoms were present. His past medical history included a lung resection of the right apical lobe due a cancer 11 years before, without adjuvant chemotherapy. Moreover, he was suffering with diabetes and blood hypertension treated with metformin and irbesartan, respectively.
At videofluoroscopic swallow examination, the surface of mucosa appeared irregularly nodular in the oropharynx, through the entire esophagus, as well as in the fundus and gastric body (Fig. 1). Upper endoscopy was promptly performed which perfectly confirmed the radiologic findings (Fig. 2). In detail, nodular lesions were detected between tongue origin and epiglottis, and diffuse submucosal nodules, with a diameter varying from 3 to 8 mm, through the entire esophagus. Normal intervening mucosa was noted. Similar nodules were observed in the gastric body and fundus, whilst the mucosa of gastric antrum, duodenal bulb and descending portion of duodenum was macroscopically normal. Multiple biopsies were taken in the esophagus, stomach, as well as in the duodenum.
Figure 1.
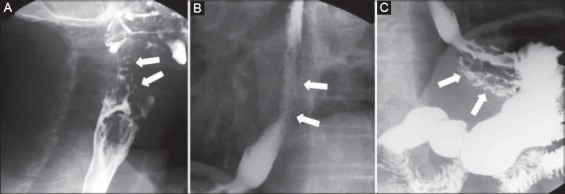
(A) Radiologic study showing grossly nodules in the oropharynx; (B) through the esophagus; (C) in gastric fundus
Figure 2.
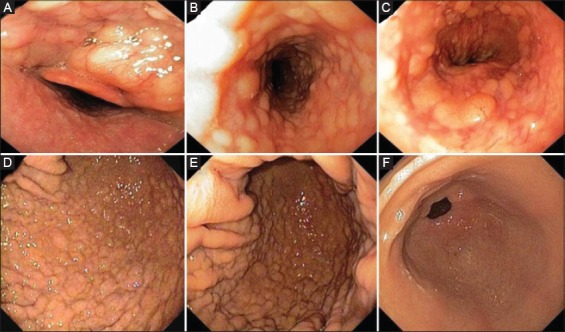
(A) Upper endoscopy confirming the radiological findings with submucosal nodules upper the epiglottis; (B) in the medium esophagus; (C) in the cardia region; (D) fundus; (E) and gastric body mucosa; (F) whilst the antral mucosa was normal
Histological assessment showed diffuse lymphoid infiltration of small lymphocytes, forming nodular aggregates, in both esophageal and gastric mucosa, and the immunohistochemistry study revealed CD20+, CD5+ and cyclin D1 overexpression (Fig. 3). The proliferative index assessment, by using the Ki67 (clone MIB-1), showed a positivity in 20% of cells. Helicobacter pylori infection was absent. Therefore, a diagnosis of MCL was made. Following a standard staging procedure, including total body computed tomography and bone marrow biopsy, a stage III disease was diagnosed with mediastinal and axillary lymph nodes involvement. Human immunodeficiency virus infection, a condition predisposing to Burkitt’s lymphoma throughout the GI tract [3], was absent. Chemotherapy including rituximab (375 mg/m2) and bendamustine (70 mg/m2) was started. Following 3 chemotherapy cycles the dysphagia markedly improved, and a repeat endoscopy showed the complete regression of esophageal lesions with an evident reduction in the nodular pattern of gastric body mucosa (Fig. 4). Chemotherapy is ongoing.
Figure 3.
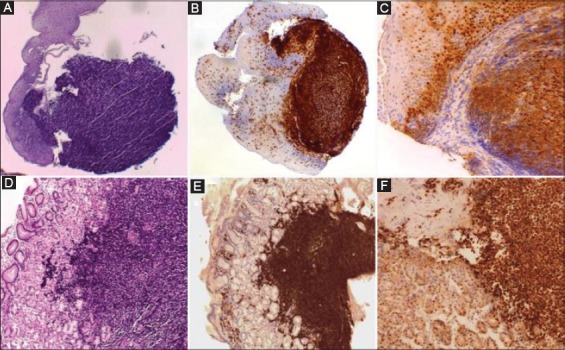
Histology revealed a mantle cell in both lymphoma esophagus (A) and stomach (D) (H&E, 20×) with the respective immunohistochemistry for CD20 (B and E) (10×), and cyclin D1 (C and F) (40×)
Figure 4.
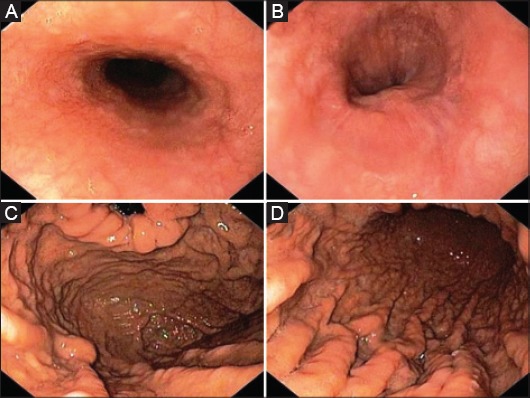
Upper endoscopy following 3 chemotherapy cycles showing regression of submucosal nodules in both the medium (A), and distal (B) esophagus, with a marked improvement in the nodular pattern of both fundus (C), and gastric body mucosa (D)
Discussion
Although GI tract is the most frequent site of extranodal lymphomas, esophageal lymphomas are very uncommon [4]. For instance, a recent case series found that only 1 case among 883 esophageal tumors observed at a Department of Surgical Oncology was a malignant centroblastic lymphoma [5]. A literature review published on 2002 found a total of 16 case reports of primary esophageal lymphoma, none of which was an MCL [6].
MCL is a rare primary non-Hodgkin’s B-cell lymphoma which may localize along the entire GI tract, where it accounts for 4-9% of all GI lymphomas [7]. However, colon and rectum were the most commonly affected parts, followed by the small bowel, stomach and duodenum [7]. Indeed, MCL of the esophagus is extremely rare. A retrospective study collecting data of 35 MCL patients with GI involvement, found that the esophagus was affected in only 2 (5.7%) cases [8]. Further 3 cases were described in whom the esophageal involvement was as a part of diffuse GI localization until the colon (stage IV disease) [9,10]. To our knowledge, the endoscopic feature of MCL has been reported in only 3 cases, and the described multiple whitish polypoid lesions in the esophagus were consistent with our finding [8,11]. Of note, such a macroscopic appearance is definitely different from that of primary low-grade, B-cell mucosa-associated lymphoid tissue (MALT) lymphoma (i.e. the marginal zone MALT lymphoma), generally confined in the stomach [12]. Indeed, in the only 2 cases described [6,13], MALT lymphoma of the esophagus presented either as a single large submucosal mass or as two slightly elevated submucosal lesions at endoscopy. Conversely, esophageal MCL presented with multiple submucosal nodules in our case. Not surprisingly, the intestinal MCL was formerly called ‘Multiple Lymphomatous Polyposis’, due to its macroscopic feature varying from submucosal nodules of few millimeters to large pseudopolyps of several centimeters [7]. Likewise, the diffuse and marked involvement of esophageal wall by MCL could explain the onset of dysphagia in our patient, whilst such a symptom was absent in the two cases of esophageal MALT lymphoma [5,11].
In conclusion, we reported a case of esophageal and gastric MCL involvement presenting with dysphagia, and we provided its peculiar radiologic and endoscopic features.
Biography
Nuovo Regina Margherita Hospital, Rome; Santo Spirito Hospital, Rome; Riuniti Hospital, Foggia, Italy
Footnotes
Conflict of Interest: None
References
- 1.Torresan F, Ioannou A, Azzaroli F, Bazzoli F. Treatment of achalasia in the era of high-resolution manometry. Ann Gastroenterol. 2015;28:299–306. [PMC free article] [PubMed] [Google Scholar]
- 2.Inoue H, Kaga M, Ikeda H, et al. Magnification endoscopy in esophageal squamous cell carcinoma:a review of the intrapapillary capillary loop classification. Ann Gastroenterol. 2015;28:41–48. [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho JR, Carrilho-Ribeiro L, Zagalo A, Cota Medeiros F, Ferreira C, Velosa J. Rare case of Burkitt’s lymphoma of the duodenal bulb. Ann Gastroenterol. 2016;29:230–232. doi: 10.20524/aog.2016.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruskoné-Fourmestraux A, Delmer A, Lavergne A, et al. Multiple lymphomatous polyposis of the gastrointestinal tract:prospective clinicopathologic study of 31 cases. Groupe D’etude des Lymphomes Digestifs. Gastroenterology. 1997;112:7–16. doi: 10.1016/s0016-5085(97)70212-9. [DOI] [PubMed] [Google Scholar]
- 5.Zielinski J, Kruszewski WJ, Jaworski R, et al. Rare oesophageal tumours:experience of one centre. Eur Surg. 2012;44:361–365. doi: 10.1007/s10353-012-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosaka S, Nakamura N, Akamatsu T, et al. A case of primary low grade mucosa associated lymphoid tissue (MALT) lymphoma of the oesophagus. Gut. 2002;51:281–284. doi: 10.1136/gut.51.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruskoné-Fourmestraux A, Audouin J. Primary gastrointestinal tract mantle cell lymphoma as multiple lymphomatous polyposis. Best Pract Res Clin Gastroenterol. 2010;24:35–42. doi: 10.1016/j.bpg.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Iwamuro M, Okada H, Kawahara Y, et al. Endoscopic features and prognoses of mantle cell lymphoma with gastrointestinal involvement. World J Gastroenterol. 2010;16:4661–4669. doi: 10.3748/wjg.v16.i37.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto Y, Nakamura N, Kuze T, Ono N, Abe M. Heterogenous group that includes mantle cell lymphoma and follicular lymphoma:analysis of somatic mutation of immunoglobulin heavy chain gene variable region. Hum Pathol. 1999;30:581–587. doi: 10.1016/s0046-8177(99)90205-8. [DOI] [PubMed] [Google Scholar]
- 10.Santa G. Oesophageal involvement in mantle cell lymphoma. Singapore Med J. 2010;51:e201. [PubMed] [Google Scholar]
- 11.Shen KH, Chen CJ, Yen HH. Multiple polyposis of the esophagus:mantle cell lymphoma. Clin Gastroenterol Hepatol. 2012;10:e65. doi: 10.1016/j.cgh.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Zullo A, Hassan C, Ridola L, Repici A, Manta R, Andriani A. Gastric MALT lymphoma:old and new insights. Ann Gastroenterol. 2014;27:27–33. [PMC free article] [PubMed] [Google Scholar]
- 13.Shim CS, Lee JS, Kim JO, et al. A case of primary esophageal B-cell lymphoma of MALT type, presenting as a submucosal tumor. J Korean Med Sci. 2003;18:120–124. doi: 10.3346/jkms.2003.18.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]


