Fig. 2.
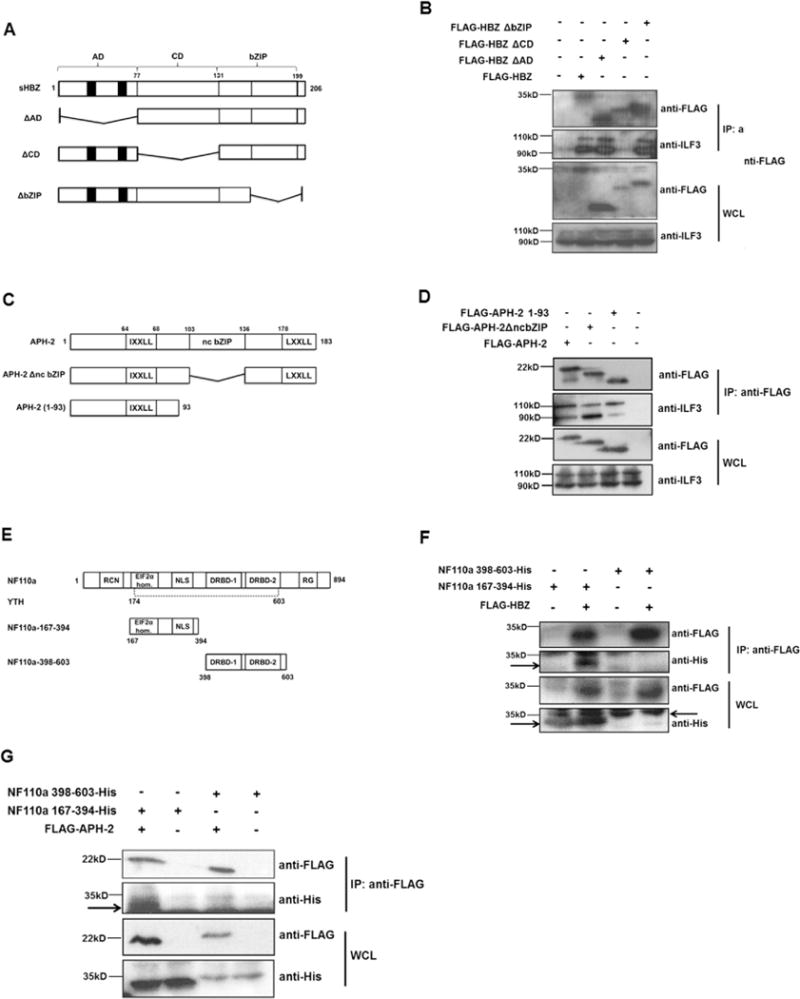
Deletion mapping of HBZ, APH-2 and NF110a interaction domains. (A) Schematic of HBZ and deletion mutants used in co-immunoprecipitations. Functional domains are: AD: activation domain; CD: central domain; bZIP: basic leucine zipper. Black boxes represent the LXXLL motifs. (B) NFARs interact with HBZ central domain. 293T cells were seeded into 60 mm cell cultures dishes and transfected with 8 μg of pFLAG-HBZ or pFLAG-HBZΔAD and 10 μg of pFLAG-HBZΔCD or pFLAG-HBZΔbZIP as indicated. 24 hours post-transfection cells were lysed in RIPA buffer containing 50 mM Tris–HCl, 150 mM NaCl, 1% Triton® X-100, 0.1% SDS, 0.5% sodium deoxycholate. An anti-FLAG M2 resin was used to precipitate protein complexes from lysates overnight. Interactions were analysed by western blot using anti-FLAG and anti-ILF3 antibodies. (C) Schematic of APH-2 and mutants used in co-immunoprecipitations. Functional domains are: IXXLL: LXXLL-like motif; ncbZIP: non-canonical basic leucine zipper domain; LXXLL: LXXLL motif. (D) 293T cells were seeded into 60 mm cell cultures dishes and transfected with 8 mg of full length APH-2 or the indicated mutants. Lysates from transfected cells were prepared using a buffer containing 1X TBS, 0.005M EDTA, 1% Triton-X 100 24 h post-transfection and subjected to immunoprecipitation using FLAG M2 resin. Precipitates were analysed by western blot using anti-FLAG and anti-ILF-3 antibodies. (E) Schematic representation of NF110a and mutants used in co-immunoprecipitations. Mutants are: NF110a-167-394 incorporating the EIF2α-homology region and NLS; 398–603 incorporating two double-stranded RNA-binding domains (DRBD-1/-2). Y2H represents the minimum domain in NF110a required for interaction with APH-2 obtained from yeast two-hybrid screen. (F and G) HBZ and APH-2 interact with NF110a 167-394. 293T cells were seeded into 60 mm cell cultures dishes and co-transfected with 2 μg pCAGGS-NF110a-167-394-His or 8 μg pCAGGS-NF110a-398-603-His and 4 μg pFLAG-HBZ (F) or pFLAG-APH-2 (G) as indicated. Cells were lysed in 1X TBS, 0.005M EDTA, 1% Triton-X 100 buffer as above. Interactions were analysed by immunoblotting using anti-FLAG and anti-His antibodies. Arrows indicate specific protein bands.
