Table 2.
Enantioselective iridium catalyzed reductive coupling of branched allylic acetates 1a–1o with formaldehyde to form primary homoallylic alcohols 2a–2o.a
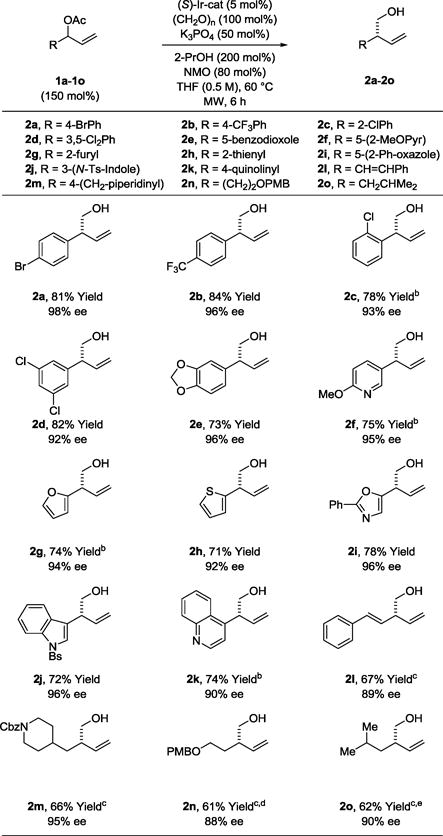
|
Yields are of material isolated by silica gel chromatography. Enantioselectivities were determined by chiral stationary phase HPLC analysis. 2k, 8 h. 2l, 10 h. See Supporting Information for further experimental details.
80 °C.
70 °C.
The catalyst modified by (S)-DM-SEGPHOS was used.
Isolated as the 4-nitrobenzoate due to volatility.
