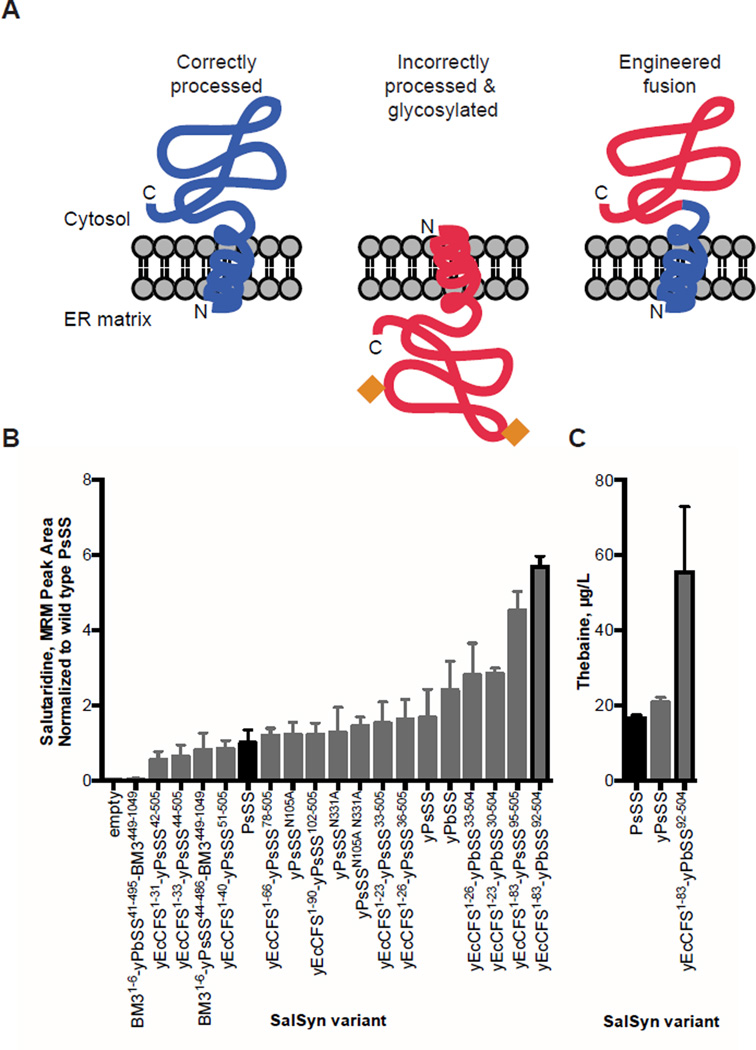
Fig. 3. Engineered SalSyn chimeras improve conversion of (R)-reticuline to salutaridine.
(A) Schematic of the chimeric SalSyn engineering strategy to address incorrect processing and glycosylation of the wild-type SalSyn in yeast. Orange diamonds represent glycosylation. (B) Comparison of salutaridine produced from SalSyn variants, site-directed glycosylation mutants, and engineered fusions in yeast. Yeast strains expressing the indicated SalSyn variant were fed 10 µM (R)-reticuline, and the growth media was analyzed by LC-MS/MS MRM. Peak areas were normalized to wild-type SalSyn (black). (C) Comparison of thebaine produced from SalSyn variants in yeast. Yeast strains were fed 1 mM rac-norlaudanosoline, and thebaine in the growth media was quantified by LC-MS/MS MRM with an external standard curve. Bars outlined in black denote wild-type and best engineered variant. Error bars are standard deviation of at least three biological replicates.