Figure 5. Putative CICs traits in CAR+/mPSCsOct-4_hi.
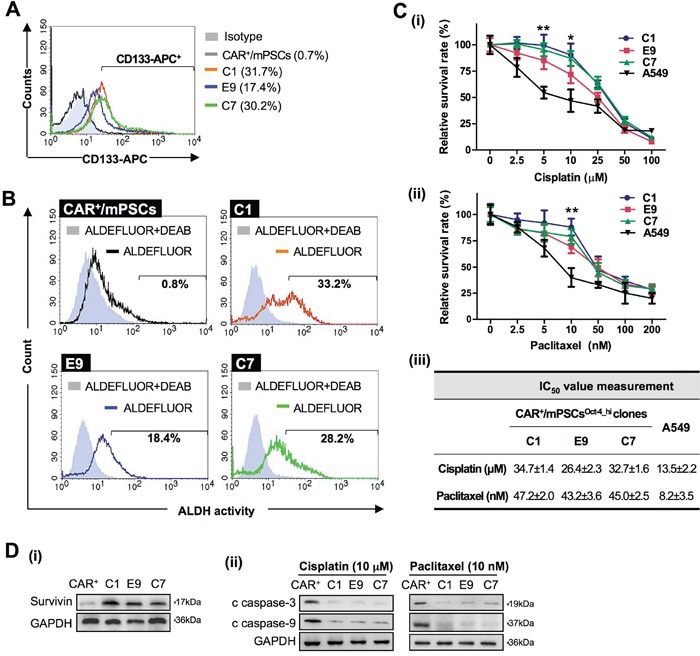
A. Flow cytometry analysis of CD133 expression in CAR+/mPSCs and CAR+/mPSCsOct-4_hi C1, E9, and C7 clones. Percentages indicate the CD133-positive population for each clone. B. Flow cytometry analysis of ALDH activity. The results of the ALDEFLUOR assay with CAR+/mPSCs and CAR+/mPSCsOct-4_hi C1, E9, and C7 clones are shown. DEAB-treated samples served as negative controls. Percentages indicate the ALDH-positive population for each clone. C. Cell viability of CAR+/mPSCsOct-4_hi. CAR+/mPSCsOct-4_hi C1, E9, and C7 clones and A549 cells were treated with (i), cisplatin (2.5, 5, 10, 25, 50, and 100 μM) or (ii), paclitaxel (2.5, 5, 10, 50, 100, and 200 nM) for 48 h. Data are shown as the mean ± SD. * P < 0.05, ** P < 0.01 compared with A549 cells. (iii), IC50 of cisplatin and paclitaxel for CAR+/mPSCsOct-4_hi C1, E9, and C7 clones and A549 cells. Data are shown as the mean ± SD. D. Anti-apoptosis potential of CAR+/mPSCsOct-4_hi clones. (i), Survivin expression in CAR+/mPSCs and CAR+/mPSCsOct-4_hi C1, E9, and C7 clones was analyzed using Western blot. (ii), Cleaved caspase-3 (c caspase-3) and cleaved caspase-9 (c caspase-9) levels in CAR+/mPSCs and CAR+/mPSCsOct-4_hi C1, E9, and C7 clones after 10 μM cisplatin or 10 nM paclitaxel treatment.
