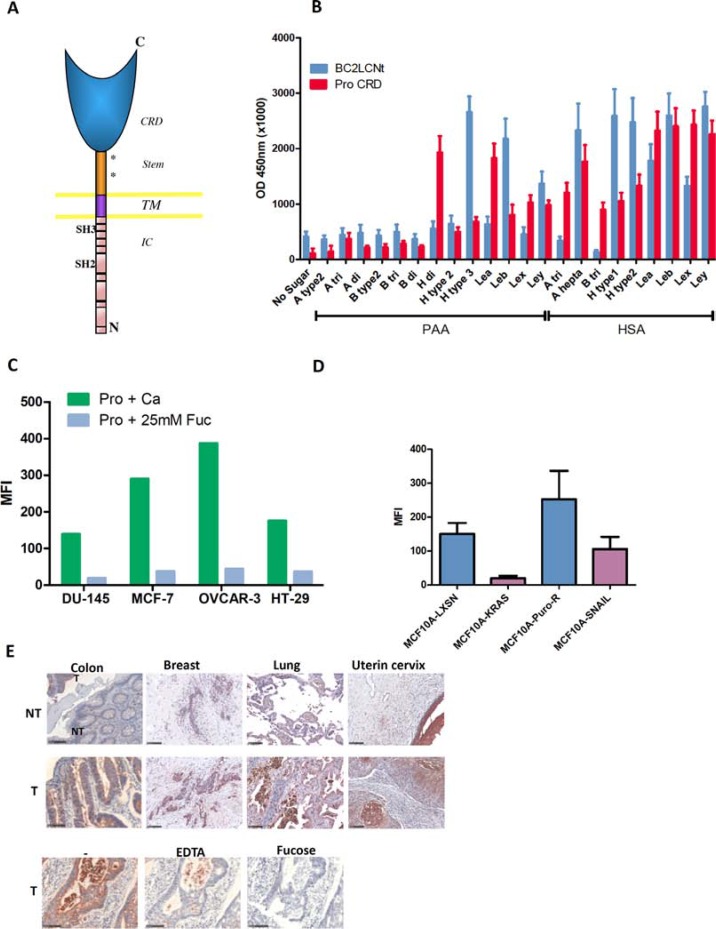
Figure 4. Prolectin is a human C-type lectin that recognizes similar fucosylated ligands as BC2L-C-Nt and efficiently binds to tumor cell lines and tumor tissues.
(A) Diagram of Prolectin structure (CRD: carbohydrate recognition domain; TM: transmembrane domain; IC: intra-cytoplasmic domain). (B) Glycan binding assay of prolectin and BC2L-C-Nt. Plates coated with a series of fucosylated HBGAs conjugated to HSA or PAA were probed with either biotinylated-BC2L-C-Nt followed by Avidin-HRP or tetramers of biotin-prolectin CRD bound to streptavidin-HRP. The results shown are the mean and SEM of three independent experiments. (C) Flow cytometry staining of tumor cell lines from various origins. Tetramers of biotinylated-prolectin CRD in complex with streptavidin-PE, pre-incubated in the absence of sugar or with L-fucose, were bound to the indicated cell lines, which were then analyzed by flow cytometry. The bar histogram shows the mean fluorescence intensities. Results are representative of at least two experiments. (D) Prolectin preferentially stains epithelial cell lines. The epithelial/mesenchymal cell lines pairs MCF10A-LXSN/MCF10A-Kras(v12) and MCF10A-PuroR/MCF10A-SNAIL were stained with prolectin as described above. Means and SEM of three independent experiments are shown (E) Prolectin binding to tumor tissues. Tetramers of biotinylated-prolectin CRD conjugated with streptavidin-HRP were incubated on fixed tissue sections from different tumor (T) or adjacent normal (NT) tissues. To control for the involvement of specific glycan binding in the prolectin staining, colon tumor sections were stained as above after prolectin tetramers were incubated with Ca2+, EDTA or Ca2++ L-fucose. Colon tissues had been fixed in ethanol whereas breast, lung and uterine tissues were from formalin-fixed TMAs. Magnifications of 20× are shown (the black bars represent 100 μm).