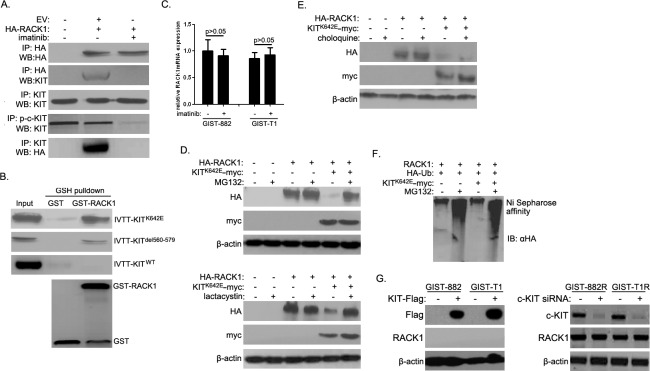
Figure 3. Activated c-KIT binds to RACK1 and is required for ubiquitin-proteasome degradation of RACK1.
A. After 48 h of imatinib treatment, whole cell lysates were prepared from GIST-T1 cells transfected with empty vector (EV) or HA-RACK1. Immunoprecipitation (IP) was performed using anti-HA, anti-p-c-KIT, or anti-KIT antibodies. Immunoblotting assays were used to detect HA-RACK1, p-c-KIT and c-KIT in whole cell lysates and IP products. B. Purified GST or GST-RACK1 was incubated with in vitro transcribed and translated wild-type, K642E, or del580-570 mutants of c-KIT, captured on glutathione (GSH)-Sepharose beads, and analyzed by SDS-PAGE followed by autoradiography and an immunoblotting assay with anti-GST. C. RACK1 mRNA levels were assessed by quantitative PCR in GIST-882 and GIST-T1 cells with or without 24 h of imatinib treatment. D. HEK293 cells were transfected with pcDNA3.0/RACK1-HA with or without pcDNA3.0/KITK642E-myc. 36 h after transfection, cells were treated with MG132 (10μM; upper panels) or lactacystine (10μM; lower panels) for another 5 h. Cell lysates were then subjected to Western blot using anti-HA or anti-myc antibody. E. HEK293 cells were transfected as in (D). 24 h after transfection, cells were treated with choloquine (10mM) for another 12 h. Cells lysates were then analyzed as in (D). F. HEK293 cells were transfected with pcDNA3.0/RACK1, pcDNA3.0/KITK642E-myc, and pcDNA3/HA-ubiquitin constructs. 36 h after transfection, cells were treated with 10μM MG132 for another 5 h. Cell lysates were subjected to nickel agarose purification. The affinity-precipitated complexes were separated and blotted with anti-HA antibody. G. pCDNA3.0-KIT-Flag was transfected into GIST882 and GIST-T1 cells, while c-KIT siRNA was transfected into GIST882R and GIST-T1R cells. 48 h after transfection, cell lysates were subjected to Western blot using anti-Flag, anti-c-KIT, or anti-RACK1 antibodies.