Abstract
In vitro models are essential to understanding the molecular characteristics of colorectal cancer (CRC) and the testing of therapies for CRC. Many efforts to establish and characterize primary CRC cell lines have been published, most describing a small number of novel cell lines. However, there remains a lack of a large panel of uniformly established and characterized cell lines. To this end we established 20 novel CRC cell lines, of which six were derived from liver metastases. Genetic, genomic and transcriptomic profiling was performed in order to characterize these new cell lines. All data are made publically available upon publication.
By combining mutation profiles with CNA and gene expression profiles, we generated an overall profile of the alterations in the major CRC-related signaling pathways. The combination of mutation profiles with genome, transcriptome and methylome data means that these low passage cell lines are among the best characterized of all CRC cell lines. This will allow researchers to select model cell lines appropriate to specific experiments, facilitating the optimal use of these cell lines as in vitro models for CRC. All cell lines are available for further research.
Keywords: colorectal cancer cell line, chemosensitivity, next generation sequencing, copy number profiling, gene expression
INTRODUCTION
With over a million cases diagnosed every year, colorectal cancer (CRC) is the third most commonly occurring cancer in the world (Globocan 2012). The occurrence of metastatic disease has a major impact on patient survival: CRC patients presenting with distant metastases have a 5-year survival rate of only 12%, while patients with local disease or regional spreading of disease show 5-year survival rates of 90% and 70%, respectively [1].
One third of CRC cases already show distant metastases at diagnosis and around 50-60% of these metastases are found in the liver [2–4]. Metastatic CRC (mCRC) is often treated using the FOLFOX protocol, a series of chemotherapy treatments consisting of oxaliplatin and 5-FU. mCRC without RAS mutations are treated with EGFR inhibition therapies such as cetuximab and panitumumab. Patients without RAS mutations show a median overall survival of 23.8 months on this treatment [5]. Patients with RAS mutant CRC are ineligible for EGFR inhibition therapy and show a median overall survival of 19.2 months.
Development of new drugs for the treatment of cancer starts with in vitro testing of candidate compounds. The availability of a cell line model that closely resembles the tumor subtype under investigation is therefore pivotal. Many well characterized cell lines exist which represent most of the CRC subtypes [6, 7]. These CRC cell lines, such as HCT116, HT-29, SW480 and LoVo, were established several decades ago [8–11]. For many of these cell lines clinicopathological parameters and information on patient characteristics are incomplete. More importantly, these cell lines have been in culture for decades and have likely diverged from initial cultures at both the genetic and epigenetic levels. This creates the concern that these cell lines might be less suitable for pharmacological testing as representative CRC models [12, 13]. Lange et al. for example state: “In contrast to cell lines of high passage, low-passage cancer cell lines well reflect the biology of the original tumor, such as growth behavior, morphology, and mutational profile and are, therefore, in our experience, a versatile tool to evaluate drug efficiencies in a preclinical context”, emphasizing that low passage cell lines are pivotal for pre-clinical drug screening [12].
Several studies were performed to establish and characterize low passage CRC cell lines. For example Maletzki et al. established CRC cell lines from 5 tumors, and extensively characterized their morphology, growth kinetics, and molecular profile [14, 15]. Several other publications describe the characterization of single low passage CRC cell lines [13, 16–18]. While an important contributions to the field, these studies all focus on various different aspects of cell line characterization and chemosensitivity. However, a uniform and comprehensive molecular characterization of low passage CRC cell lines is lacking.
For this reason we sought to generate novel CRC cell models and have now established a panel of 20 new CRC cell lines. Six of these originated from CRC liver metastases, while the remaining cell lines all derived from primary CRC tumors. We performed characterization of these novel CRC cell lines, including somatic mutation profiling genomic and transcriptomic analyses. Additionally, sensitivity to oxaliplatin was tested as a measure of sensitivity to current CRC treatment regimens. The combined dataset are publically available. These novel CRC cell lines will serve as a valuable research tool in addition to currently available cell lines to be used for in vitro drug research and may help further understanding of the molecular mechanisms underlying CRC.
RESULTS
Here we report the establishment of 20 novel CRC cell lines, 14 of which were derived from primary colorectal cancers, while the remaining 6 were established from liver metastases. To assure these cell lines are permanent and stable, the cell lines were cultured for at least 30 passages. For the analyses described here cultures of approximately 13 passages were used. All cell lines have successfully been cultured multiple times from frozen vials to ensure they are able to survive the freezing process. The recovery rate was between 60-90%, except for JVE774 (10%).
We performed comprehensive genomic profiling of the cell lines, including genome-wide gene expression, copy number and somatic mutation analyses.
Clinicopathological characteristics
Of the cell lines derived from primary tumors, five originated from distal CRC, including rectal and sigmoid tumors, while 9 originated from proximal CRC tumors, amongst others from the cecum and the ascending colon. The various tumor locations are listed in Table 1. Six of the 20 cell lines were derived from CRC liver metastases. Histological classification of the tumors from which these cell lines were derived were extracted from the pathology report. The majority of primary tumors were colorectal adenocarcinomas, including 4 mucinous adenocarcinomas.
Table 1. Cell line characteristics and mutation profiles.
| Cell line | Age | Gender | Location | Tumor morpho-logy | Dukes stage |
Cell line growth |
MSI | BRAF | KRAS | PIK3CA | TP53 | APC | SMAD4 | Other | MLH1 | MGMT | CDKN2A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JVE015 | 66 | F | S | -- | -- | Monolayer | MSS | -- | c.34G>T | c.3140A>G | c.916C>T | c.3340C>T | -- | -- | U | M | M |
| JVE044 | 83 | F | R | -- | -- | Piled-up | MSS | -- | c.35G>T | -- | c.524G>A | -- | c.931C>T | FBXW7: c.1513C>T | U | U | M |
| JVE059 | 58 | M | T | AC | D | Monolayer | MSI-H | -- | -- | -- | -- | -- | -- | EGFR: c.2164G>A MLH1: c.551C>A MLH1: c.1975C>T | U | U | P |
| JVE103 | 51 | M | Meta | AC | D | Piled-up | MSS | -- | -- | -- | -- | -- | -- | SMAD3: HomDel | U | U | U |
| JVE109 | 84 | F | H | AC | C1 | Monolayer | MSI-H | c.1799T>A | -- | -- | c.842A>G | -- | -- | VHL: c.449A>G FGFR2: c.544G>A | M | U | M |
| JVE114 | 71 | M | Meta | AC | D | Monolayer | MSS | -- | -- | -- | c.638G>T | c.3921_3925del | -- | -- | U | U | U |
| JVE127 | 60 | M | A | MAC | C2 | Monolayer | MSS | c.1799T>A | -- | -- | c.394A>C | -- | -- | PTEN: HomDel | U | U | U |
| JVE187 | 60 | F | Meta | AC | D | Monolayer | MSS | -- | c.351A>T | c.1636C>A | -- | c.4668_4669del | c.1082G>A | ERBB2: c.2524G>A | U | M | M |
| JVE192 | 43 | F | A | MAC | C1 | Monolayer | MSI-H | -- | c.38G>A | c.3062A>G | c.743G>A | -- | -- | MLH1: c.112A>C FGFR3: c.1906G>A CTNNB1: c.121A>G GNAS: c.601C>T | U | U | P |
| JVE207 | 67 | M | D | AC | C2 | Monolayer | MSS | c.1799T>A | -- | -- | c.527G>A | -- | HomDel | -- | U | U | U |
| JVE222 | 67 | F | A | AC | B2 | Monolayer | MSI-H | -- | c.38G>A | c.263G>A | -- | c.4348C>T | -- | MSH6: c.2718_ 2719del | U | U | P |
| JVE241 | 79 | M | C | MAC | B2 | Piled-up | MSS | -- | c.35G>T | -- | c.257_279del | c.4496G>T | -- | -- | U | U | U |
| JVE253 | 48 | F | Meta | MAC | D | Monolayer | MSS | -- | c.35G>T | -- | c.742C>T | c.4709_4713del | -- | -- | U | U | P |
| JVE367 | 61 | F | I | LNEC | C1 | Suspension | MSS | c.1799T>A | -- | -- | -- | -- | -- | -- | U | U | M |
| JVE371 | 67 | M | Meta | AC | D | Monolayer | MSS | -- | c.34G>A | c.1633G>A | c.673-1G>A | -- | -- | -- | U | M | U |
| JVE528 | 57 | F | A | AC | B1 | Piled-up | MSS | -- | c.38G>A | -- | c.1024C>T | c.4348C>T | -- | -- | U | M | P |
| JVE774 | 61 | M | R | AC | B1 | Monolayer | MSS | -- | c.64C>A | -- | -- | c.4033G>T | -- | -- | U | P | P |
| KP283T | 49 | F | Meta | AC | D | Monolayer | MSS | -- | c.34G>T | -- | c.818G>A | -- | -- |
ALK: c.3599C>T PTEN: HomDel |
U | U | U |
| KP363T | 80 | M | Ls | AC | B2 | Monolayer | MSS | c.1799T>A | -- | c.1633G>A | c.916C>T | -- | HomDel | -- | U | U | M |
| KP7038T | 34 | M | Rs | AC | B1 | Monolayer | MSI-H | -- | -- | -- | -- | c.3292_3293del | -- | MLH1: exon16 HomDel | U | U | U |
Abbreviations: A = Ascending colon, C = Cecum, D = Descending colon, H = Hepatic flexure, i = Ileocecal junction, Ls = Left-sided colon, Meta = Liver metastasis, R = Rectum, Rs = right-sided colon, S = sigmoid, T = Colon Transversum, AC = AdenoCarcinoma, MAC = Mucinous AdenoCarcinoma, LNEC = Largecell Neuro-Endocrine Carcinoma, U = unmethylated, M = methylated, P = Partially methylated
Line JVE367 was derived from a large-cell neuroendocrine carcinoma (LNEC). LNEC account for only 0.2% of all CRC, and generally are associated with a very poor prognosis. [19, 20] To our knowledge JVE367 is the first LNEC cell line of colonic origin and offers an in vitro model to study this aggressive CRC subtype.
Cell line morphology and identity
Considerable differences in cell morphology could be observed between the cell lines. The various morphologies are illustrated in Figure 1 and growth characteristics are given in Table 1. Morphologically, two groups can be distinguished, with cells growing either in a monolayer or piled-up. Cells growing in monolayers consist of stretched cells, which grow as non-overlapping islands of cells (Figure 1A; JVE222 and 1B: JVE253). JVE059 and JVE127 also grow in monolayers but appear to be less adherent and form a layer of rounded, single cells (1C and 1D, respectively). The cell lines with a piled-up morphology form clumps of multi-layer cells, interspersed with cells in monolayer. Examples are shown in 1E (JVE528) and 1F (JVE044). Two cell lines, JVE367 and JVE241, show more unusual growth patterns. JVE367 (Figure 1G) grows in suspension, both in clumps and as single cells, and remains in suspension even when cells are transferred to collagen or gelatin-coated culture flasks. JVE241 (Figure 1H) shows a piled-up morphology, without cells that form a monolayer between the piled-up islands. Representative images of the other 12 cell lines are included in Supplementary Figure S1. Cell line identity was analyzed using short tandem repeat (STR) profiles (Supplementary Table S2). All cell lines are unique, and do not match any known cell line in the DSMZ database.
Figure 1. Examples of different cell line morphologies.
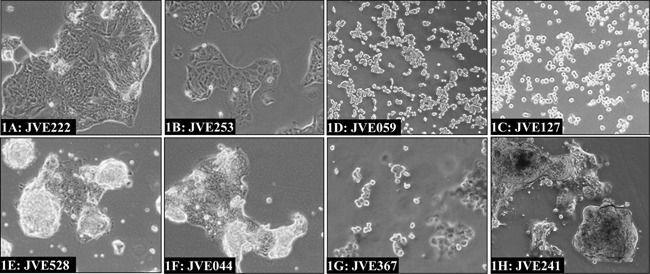
The predominant morphological growth patterns among the newly established cell lines include islands of cells in monolayers as illustrated by JVE222 and JVE253 1A. and 1B. a rounded off morphology as seen for JVE127 and JVE059 1C. and 1D. and cells with a piled-up morphology interspersed with monolayers, of which JVE528 1E. and JVE044 1F. are examples. JVE367 1G. is the only cell line that grows in suspension, while JVE241 1H. grows in a piled-up fashion but lacks the monolayers typical of the other cell lines with piled-up morphology.
Sensitivity to chemotherapeutics
As a measure of resistance to chemotherapeutic regimens used in the treatment of CRC, sensitivity to oxaliplatin was assessed. Cell lines fell into one of three sensitivity groups; a resistant group (IC50 > 25 μM, N = 4), the high sensitivity group with IC50's below 10 μM (N = 7) and an intermediate group with IC50's between 10 μM and 25 μM (N = 9). Dose-response curves for JVE059 and KP283T are shown in Figure 2A and 2B as examples of sensitive and resistant cell lines. As shown in Figure 2C, cell lines derived from liver metastases showed a lower sensitivity to oxaliplatin (t-test, p < 0.02). IC50 values for oxaliplatin, including 95% confidence intervals, are included in Supplementary Table S1.
Figure 2. Oxaliplatin resistance.
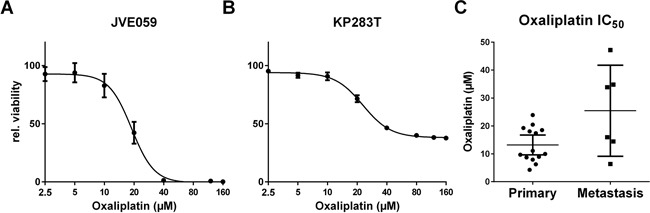
Dose-response curves were generated for all cell lines in order to determine IC50 values. Examples are shown for JVE059 A. and KP283T B. with the latter showing high oxaliplatin resistance. C. shows the IC50 values grouped in either primary or metastasis-derived cell lines, and indicates an enrichment of resistant cell lines amongst the metastasis-derived cell lines (p < 0.02). Bars represent the mean, with 95% confidence intervals.
DNA mismatch repair deficiency
While only 15% of primary CRC tumors show MSI-H [21] these cell lines are overrepresented in the available collections. In the Seltar database (www.seltarbase.org, accessed October 5th 2015) 73 out of 147 CRC cell lines are MSI-H and recently Medico shows 41,7% of cell lines are MSI-H [7]. Five of our 20 cell lines (25%) were found to be MSI-H, all of which originate from tumors of the proximal colon. In sporadic CRC, MSI is most often caused by hypermethylation of the MLH1 promoter, resulting in loss of the MutL complex. Therefore, MLH1 DNA promoter hypermethylation was assessed. JVE109 was found to be hypermethylated at the MLH1 promoter, while hypermethylation was absent in all other cell lines.
Amongst the most frequent targets in MSI-H tumors are the TGF-β and activin receptors, TGFBR2 and ACVR2A. The microsatellites in exon 3 of TGFBR2 and the 3rd and 10th exons ACVR2A were screened in all cell lines (Supplementary Table S3). Mutations in the TGFBR2 microsatellite and in the 10th exon of ACVR2A were found in four MSI-H cell lines. None of the cell lines showed instability of the microsatellite in exon 3 of ACVR2A, in concordance with previous results [21]. The fifth MSI-H cell line (JVE222) did not show instability at any of the TGFBR2 and ACVR2A microsatellites.
CDKN2A and MGMT promoter hypermethylation
In addition to MLH1, promoter hypermethylation of the CDKN2A and MGMT promoters was also assessed using methylation specific PCR (MSP). Hypermethylation of MGMT is observed in 38% [22] and CDKN2A methylation is found in 25% of CRCs [23]. In CRC cell lines, CDKN2A is methylated in up to 75% of cases [24, 25], and CDKN2A hypermethylation has been proposed to be part of a cell line-specific DNA methylation pattern [24]. MSP identified 5 cell lines that were hypermethylated for MGMT: of these only JVE774 showed partial methylation. MSP of CDKN2A identified 12 cell lines with hypermethylation of CDKN2A, of which 6 cell lines were partially methylated. Partial hypermethylation can be explained by either methylation of one of the alleles or by a mixed population of cells with and without hypermethylation at these loci. A more accurate quantification of the percentage of methylated alleles could shed more light on this.
Somatic mutation profiling
To further characterize the cell lines, somatic mutations were studied in mutation hotspot regions of 50 known oncogenes and tumor suppressor genes, including KRAS, NRAS, PIK3CA and BRAF hotspots and part of the mutation cluster regions of APC and TP53. The most frequent mutated gene is TP53, with damaging mutations found in 13 cell lines.
Activating mutations in KRAS were found in 11 cell lines, including the less common KRAS activating mutations c.64C>A (p.Q22K) and c.351A>T (p.K117N). APC mutations were found in 9 cell lines.
We identified 5 cell lines with a BRAF c.1799T>A (p.V600E). Notably, all BRAF-mutated cell lines derived from primary CRC, while KRAS mutations were found in both primary and metastasis-derived cell lines. We identified PIK3CA mutations in 6 cell lines and all these cell lines also carried either a KRAS or a BRAF mutation. Co-occurrence of PIK3CA mutations with activating mutations in the mitogen activated protein kinase (MAPK) pathway was also observed in 74% of the TCGA colon adenocarcinoma samples. Damaging SMAD4 mutations were found in 2 cell lines. Additionally, next generation sequencing (NGS) data revealed homozygous deletions of SMAD4 in two additional cell lines. Similarly, PTEN homozygous deletions were observed in KP283T and JVE127, although no PTEN mutations were found. A possible activating EGFR c.2164G>A mutation as found in JVE059, however, the effect of this mutation is currently unknown. In the 4 MSI-H cell lines without MLH1 methylation, NGS of the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 was performed. MLH1 mutations were identified in JVE192 and JVE059. JVE192 carried the c.112A>C, p.N38H mutation, while JVE059 carried the compound heterozygous nonsense mutations c.551C>A and c.1975C>T. A homozygous MSH6 mutation, c.2718_2719del, p.V907Rfs*10, was found in JVE222. Analysis of KP7038T failed to show a variant, but did reveal lack of coverage of exon 16 of MLH1, suggesting the presence of a homozygous deletion. We carried out PCR analysis spanning exons 15-17 of MLH1 in KP7038T. The expected PCR product of 6497 bp was absent and was replaced by a PCR product of approximately 1350 bp. Sequencing analysis confirmed a deletion of 5146 bp at g.chr3:37084101-37089246, covering MLH1 exon 16 and resulting in an in-frame deletion of 55 amino acids. This deletion disrupts the PMS2 interaction domain, explaining the MSI-H profile of this cell line. An overview of all mutations, deletions and the promoter hypermethylation identified is provided in Table 1. The corresponding protein alterations resulting from these mutations are listed in Supplementary Table S5.
Genome and transcriptome analysis
Complementary to the mutation profiles we generated genomic and transcriptomic profiles of all cell lines by hybridizing DNA and RNA respectively to Infinium HumanExome-12v1 BeadChips. Log2 relative gene expression values are included in Supplementary File 1.
Gene expression of MLH1, PTEN and SMAD4
We evaluated the gene expression at the loci with homozygous deletions in SMAD4 and PTEN, and for promoter DNA hypermethylation of MLH1. MLH1 promoter hypermethylation was detected in JVE109. Expression of MLH1 was found to be 15-fold decreased in this sample compared to all other cell lines (Supplementary Figure S2).
The gene expression data for the samples with homozygous deletions in SMAD4, PTEN and MLH1 do not show a complete loss of gene expression for these genes. This is due to our selection of SNPs from the gene expression dataset, which are homozygous in the DNA in all samples. In the samples with a homozygous deletion, the SNP's at these sites are not called due to low signal intensity. Therefore the gene expression values reported are only based on the SNP's which are not in the deleted area's. As a result of this, PTEN expression is not included in our gene expression dataset, as the array contains only one exonic PTEN probe, which is not called homozygous in the samples with a deletion. PTEN gene expression values were extracted manually from the raw data, and plotted in Supplementary Figure S2, as well as SMAD4 expression levels.
To clarify this we plotted the average intensity in the DNA and expression datasets per probe for JVE207 (Figure 3A) and KP363T (Figure 3B), with the average intensity of all 20 cell lines in grey. Above the plot a schematic depiction of SMAD4 is given to show the location in the gene. Both samples show a clear reduction in intensity in the expression data at all loci that show a copy number loss.
Figure 3. Probe level copy number and expression intensity for SMAD4 in JVE207 and KP363T.
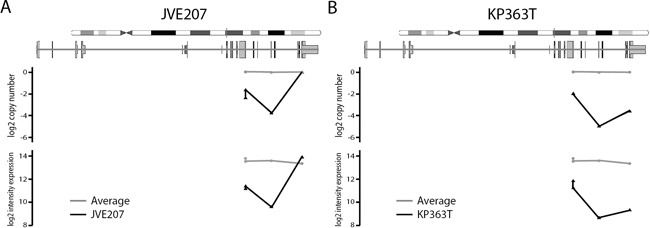
For both cell lines the copy number level and intensity in the expression data is compared to the average of all 20 cell lines. The JVE207 A. and KP363 B. data is plotted in black and the average of all 20 cell lines is shown in grey. Probes are plotted on their location in the SMAD4 gene. All probes with reduced signal in the copy number data also show a reduction in intensity in the expression data.
Alterations in CRC signaling pathways
By combining the somatic mutation profiles and gene expression data, we were able to map alterations in the main signaling pathways. A graphic display of modifications in the Wnt, BMP/TGF-β, PI3K and receptor tyrosine kinase (RTK) pathways is given in Figure 5. As our expression dataset does not contain a reference, we depict cell lines which deviate from the majority as being altered. Most cell lines showed alterations in 2 or 3 of the signaling pathways, while JVE187, JVE192 and KP363T were altered for all 4 pathways. JVE114, a liver metastasis-derived cell line, was altered for only one of the pathways, indicating that tumors with few alterations in these pathways are also capable of developing metastases. Supplementary Table S3 shows all somatic alterations and gene expression values incorporated in this overview.
Figure 5. Somatic alterations in major CRC signaling pathways leading to a decrease in differentiation and increased proliferation and cell survival.
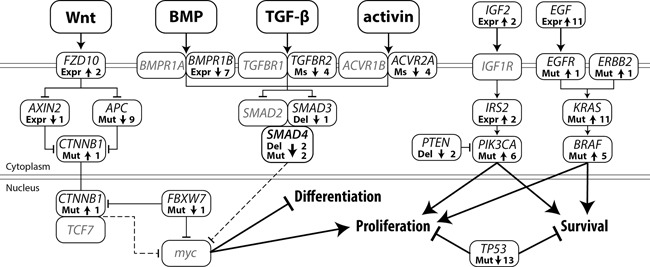
Arrow up: activating alteration, Arrow down: inactivating alteration, Expr: gene expression, Ms: microsatellite mutation, Mut: mutation, Del: deletion, Line with arrow: protein activation, Line with T-arrow: protein inhibition, Dotted line with T-arrow: inhibition of gene expression. The number represents the total number of cell lines with that specific alteration.
Upregulation of the MAPK signaling pathway is a common alteration in CRC and is generally mediated by activating mutations in KRAS, BRAF or NRAS. Alternatively, by upregulating EGF gene expression, cancer cells establish an autocrine feedback loop, abolishing their dependency on systemic EGF. Upregulation of EGF gene expression in CRC occurs in 9.7% of tumors (using a cutoff of 3x the median for normal colon samples) [26]. Upregulation of EGF was detected in 11 cases, 9 of which also showed a KRAS or BRAF mutation, and could therefore be considered to be no longer dependent on systemic EGF. JVE059 and JVE114 are the only cell lines that show neither a mutation in BRAF or KRAS, nor EGF upregulation. JVE059 harbors an EGFR mutation (c.2164G>A, p.A722T) within the tyrosine kinase domain, although it is not known whether this mutation leads to constitutive activation.
A KRAS c.351A>T, p.K117N mutation was identified in JVE187. This mutation is known to result in constitutive activation of MAPK signaling, albeit to a lower extent than mutations in codons 12, 13 and 22 [27]. Interestingly, the cell line also carries an ERBB2 mutation, c.2524G>A, which is also known to result in constitutive activation of the MAPK pathway [28].
As a result of activating mutations in PIK3CA and inactivation of PTEN, PI3K signaling was activated in half of the cell lines. Alternative routes of activation for this pathway include transcriptional upregulation of IGF2 and IRS2. JVE103 and JVE371 both showed a 4-fold increase in IGF2 expression levels compared to the other samples. IRS2 is known to be upregulated in a small proportion of CRC tumors [29]. Our dataset showed high IRS2 expression levels in almost all cell lines, although it is difficult to draw any conclusions from this without reference values. JVE371 showed substantially lower IRS2 expression levels, while it was amongs the highest IGF2 expressing cell lines. As JVE371 also carries an activating PIK3CA mutation, it is probably independent of upstream signaling. JVE103, on the other hand, is wildtype for both PIK3CA and PTEN, which suggests that this cell line may have upregulated the PI3K pathway through an autocrine upregulation of IGF2.
Metastasis-specific copy number alterations
DNA copy number profiles of each of the cell lines were generated using the Infinium HumanExome-12v1 data. In addition to copy number profiles, LOH and genomic imbalances were visualized by lesser allele intensity ratio (LAIR) analysis using the HumanExome-12v1 data [30]. Cell line copy number profiles and LAIR plots are included in Supplementary File 2.
Differences in copy number profiles between CRC metastases and primary CRC tumors have been reported previously [31]. Liver metastases and tumors that later formed liver metastases reportedly show gains of chr20q. We therefore compared the copy number profiles of the CRC liver metastasis cell lines with those of the primary CRC tumors to determine whether this alteration could also be detected in these cell lines. The frequency of gains and losses per group are shown in Figure 4A (primary CRC derived cell lines) and Figure 4B (liver metastasis derived cell lines). We found chr20q amplification in 5 of the metastasis cell lines. In addition, 50% of the primary tumor derived cell lines showed a loss of chr20. This is further evidence that chr20q amplification is highly specific for liver metastases. A frequent gain of chr20q was also found in the TCGA data [32], and the comparative study of 63 cell lines [6].
Figure 4. CNA comparison between primary and metastasis-derived cell lines.
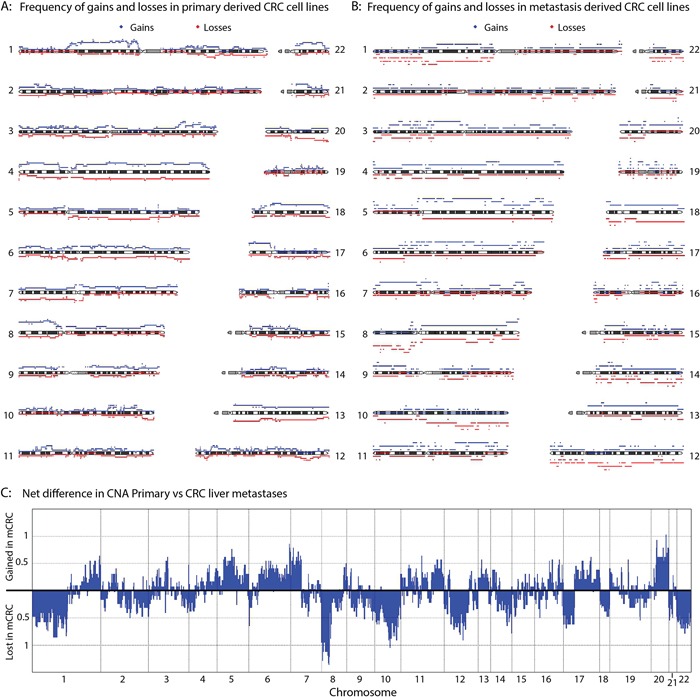
The frequency of gains (red) and losses (blue) for the primary CRC derived cell lines A. and the liver metastasis cell lines B. is plotted. C. Combining the frequency of gains and losses for each position per group, total frequencies of gains and losses in the metastasis-derived cell lines compared to the primary CRC derived cell lines was calculated.
To visualize the differences in copy number alterations (CNA) between primary tumor cell lines and metastasis-derived cell lines, the overall differences in CNA between the groups were plotted (Figure 4C). In addition to chr20q alterations, other chromosomes also show a clear difference between groups. For example, chromosomes 10 and 12 are lost predominantly in metastases, whereas chr11 is preferentially gained. Similar to the observations made concerning chr20q; chr7p was found to be lost in 40% of the primary cell lines, whereas 50% of the metastasis cell lines showed a gain of chr7q. The most marked difference between the metastases and primary tumor cell lines was CNA on chr8p. All of the liver metastasis cell lines showed a loss at chr8p, whereas around 70% of the primary cell lines showed a gain. Also both the TCGA data and a recent study of 63 cell lines showed a very high frequency of chr8p loss, similar to what we see in the metastasis cell lines[6]. The presence of metastasis-specific copy number alterations at these chromosomal locations could indicate that these regions harbor genes important for metastases.
DISCUSSION
We established 20 novel cell lines from primary and metastasized CRC tumors. Using NGS and microarray-based approaches, we generated genetic, genomic and transcriptomic profiles and evaluated cell line sensitivity to oxaliplatin as a measure for chemoresistance. Thus we established a set of low passage CRC cell lines, which have all been thoroughly characterized in a uniform manner.
The characterization of these low passage cell lines identified a large diversity in mutation spectra and gene expression profiles. This extensive panel of new CRC cell lines represents a valuable research tool that can now be applied to in vitro experiments to further untangle the complexity of CRC tumorigenesis, and to the development of new therapies for both primary and metastatic disease.
The combined dataset presented here elevates these cell lines to rank among the best characterized CRC cell lines. The SNP array data will also be made available through GEO (accession numbers GSE67773 and GSE67774), allowing researchers to select appropriate cell line models for their particular experiment, thus allowing optimal use of these novel cell lines as in vitro models for CRC. The cell lines characterized in this manuscript are deposited at the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (www.dsmz.de).
MATERIALS AND METHODS
Cell line establishment and culture
Anonymized tumor material was thoroughly rinsed using RPMI-1640 medium under vigorous tapping. The tissue was cut into ± 1 mm3 fragments which were enzymatically dissociated using a 1% collagenase I-A (Sigma), 1% dispase (Gibco Life Technologies) solution. The dissociated cells were washed with RPMI-1640 and culturing was commenced in DMEM/F12 supplemented with 10% fetal bovine serum (FBS), 100 U penicillin and 100 μg streptomycin per mL. Once the cell line was established, further culturing was performed in either RPMI-1640 or DMEM/F12 medium (lines JVE222 and JVE241) supplemented with 10% FBS, 2 mM Glutamax-I, 50 U penicillin and 50 μg streptomycin per mL (all from Life Technologies, Grand Island, NY, USA). Cells were cultured at 37°C with 9% CO2 in a humidified stove. Anonymized samples were handled according to the medical ethical guidelines described in the Code Proper Secondary Use of Human Tissue established by the Dutch Federation of Medical Sciences. All cell lines were checked for mycoplasma using a mycoplasma-specific PCR [33].
DNA isolation
DNA isolation was performed using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer's instructions.
RNA isolation
RNA isolation was performed on cells in exponential growth phase, using TRIzol® Reagent (Life Technologies). DNAse treatment was performed in suspension using rDNAse (Macherey Nagel GmbH & Co. KG, Düren, Germany).
Cell line authentication
Short tandem repeat (STR) profiles of the cell lines were established using the CellID system (Promega) according to the manufacturer's instructions. Fragment analysis was performed on an Applied Biosystems 3130 Genetic Analyser (Life Technologies). STR profiles for all cell lines are included in Supplementary Table S2.
Toxicity profiling
Cells were seeded in 96-well plates at 10000 cells per well. Twenty-four hours after seeding, the medium was removed and fresh medium containing oxaliplatin (Xeloda, Fresenius Kabi Nederland B.V., Zeist, The Netherlands) was added. After 72 hours of incubation with the compound, viability was assessed using the PrestoBlue® assay (Life Technologies) according to the manufacturer's instructions. Toxicity profiling was performed in triplicate and reproduced in 2 independent experiments. IC50 concentration was determined using Graphpad Prism® software (version 5.01).
Microsatellite analysis
The microsatellite instability (MSI) status of each of the cell lines was determined using the MSI analysis system (Version 1.2, Promega) according to the manufacturer's instructions. Fragment analysis was performed on an Applied Biosystems 3130 Genetic Analyser (Life Technologies). Samples with at least two out of five unstable mononucleotide markers were classified as MSI-H. TGFBR2 and ACVR2 microsatellite analysis was performed as described previously [21].
Methylation specific PCR
Bisulfite conversion was performed using the EZ DNA methylation gold kit (Zymo research, Orange, California, USA) according to the manufacturer's instructions, using an input of 200 ng of DNA. Bisulfite-converted DNA was eluted in 15 μL MQ water.
MLH1 and MGMT MSPs were performed according to protocols developed at the Pathology Department molecular diagnostics lab at the LUMC [34]. MGMT methylation-specific PCR (MSP) was performed using the same protocol, using the following primers: MGMT_Um_Fw: TTTGTGTTTTGATGTTTGTAGGTTTTTGT, MGMT_Um_Rev: AACTCCACACTCTTCCAAAAACAAAACA, MGMT_M_Fw: TTTCGACGTTCGTAGGTTTTCGC, MGMT_M_Rev: GCACTCTTCCGAAAACGAAACG.
For the CDKN2A, 1 μL of bisulfite converted DNA was used in combination with 1 pmol primers in a total volume of 10 μl, containing 1x IQ SYBR Green supermix (Biorad, Hercules, California, USA). The primers used were as follows: CDKN2A-M-Fw: TGTAAAACGACGGCCAGTTTATTAGAGGGTGGGG CGGATCGC, CDKN2A-M-Rev: CAGGAAACAGC TATGACCGACCCCGAACCGCGACCGTAA, CDKN2A-Um-Fw: TGTAAAACGACGGCCAGTTTATTAGAG GGTGGGGTGGATTGT, CDKN2A-Um-Rev: CAGGA AACAGCTATGACCCAACCCCAAACCACAACCA TAA. PCR protocol: 5′ at 95°C, 40 cycles of 15″ at 95°C, 30″ at 69°C and 30″ at 72°C. CDKN2A methylation status was determined by gel analysis of the PCR product and by melting curve analysis.
Somatic mutation profiling
Genomic DNA (10 ng) from each sample was used to prepare barcoded libraries using IonXpress barcoded adapters (Life Technologies). Libraries were pooled to a final concentration of 15 ng/mL after quantification with a fluorometer (Qubit HS, Life Technologies), and emulsion PCR was performed using the Ion PGM Template OT2 200 kit on a OneTouch-2 instrument. Sequencing was performed on an Ion Torrent Personal Genome Machine, using 316v2 chips.
Somatic mutations were analyzed using the Ion AmpliSeq™ Cancer Hotspot Panel v2. A list of the target genes included in this panel, along with mutation frequencies in CRC reported by the TCGA, is included in Supplementary Table S4 [29]. The pathogenicity of non-synonymous variants was assessed using PolyPhen2, MutationTaster and MutationAssessor. Mutations predicted to be damaging by at least 2 of these tools were considered to be pathogenic. Frameshift and nonsense mutations were considered to be always damaging.
Mutations in codon 600 of BRAF, codons 12 and 13 of KRAS and codons 545 and 1047 of PIK3CA were validated using Taqman genotyping assays [35].
Infinium HumanExome-12 v1 BeadChips
Infinium HumanExome-12 v1 BeadChips were used with an input of 200 ng DNA. Raw data and preprocessed intensities per probe are available via the Gene Expression Omnibus (GEO) under accession numbers GSE67773 and GSE67774. For gene expression analysis using the same platform, 500 ng of RNA was converted to cDNA using the DyNAmo™ cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA). cDNA was then purified using the QIAquick PCR purification kit (Qiagen, Germantown, Maryland, USA) and eluted in 15 μL MQ water. Five μl of purified cDNA was used as input for the Infinium protocol.
Copy number and LAIR analysis
Lesser allele intensity ratio (LAIR) analysis was performed as described previously [30]. Copy number profiles and group copy number analysis was performed using the DNAcopy package [36]. For grouped copy number differences, gains and losses were called using a threshold of 0.10 deviation from the median copy number, as applied by GISTIC2.0 [37].
Gene expression analysis
Gene expression data was generated using the intensity data of the cDNA hybridization on the Infinium HumanExome-12 v1 BeadChips. Intronic probes and probes which were heterozygous in any sample were removed. Subsequently, intensity data related to the color of the genotyped allele was extracted for each probe. After quantile normalization using the Limma package [38], the average probe intensity per gene was calculated and gene expression was reported in log2 expression values per gene per sample. In total 17090 genes were assayed. Gene expression values are included in Supplementary File 1.
SUPPLEMENTARY FIGURES AND TABLES
Acknowledgments
We would like to acknowledge Melanie Schrumpf for technical assistance.
Footnotes
CONFLICTS OF INTEREST
Authors declare no conflicts of interest.
GRANT SUPPORT
This works was supported by the EUROTRANS-BIO project FAST-SEQ.
Authors' contributions
AB, HM and TvW designed the study; AB and JDHvE performed the experiments; AB, ASPLC, DR and JO analyzed the data; JDHvE, FMS and JJC were involved in sample collection; AB and TvW wrote the manuscript. All authors reviewed and approved the final version.
REFERENCES
- 1.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldon W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, et al. SEER Cancer Statistics Review, 1975–2007. http://seer.cancer.gov/csr/1975_2007/.2010
- 2.Ismaili N. Treatment of colorectal liver metastases. World J.Surg.Oncol. 2011;9:154–154. doi: 10.1186/1477-7819-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause P, Flikweert H, Monin M, Seif Amir HA, Helms G, Cantanhede G, Ghadimi BM, Koenig S. Increased growth of colorectal liver metastasis following partial hepatectomy. Clin.Exp.Metastasis. 6-2-2013;5:681–93. doi: 10.1007/s10585-013-9572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vidal-Vanaclocha F. The liver prometastatic reaction of cancer patients: implications for microenvironment-dependent colon cancer gene regulation. Cancer Microenviron. 2011;42:163–180. doi: 10.1007/s12307-011-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, Ruff P, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann. Oncol. 2014;257:1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 6.Mouradov D, Sloggett C, Jorissen RN, Love CG, Li S, Burgess AW, Arango D, Strausberg RL, Buchanan D, Wormald S, O'Connor L, Wilding JL, Bicknell D, et al. Colorectal cancer cell lines are representative models of the main molecular subtypes of primary cancer. Cancer Res. 15-6-2014;7412:3238–3247. doi: 10.1158/0008-5472.CAN-14-0013. [DOI] [PubMed] [Google Scholar]
- 7.Medico E, Russo M, Picco G, Cancelliere C, Valtorta E, Corti G, Buscarino M, Isella C, Lamba S, Martinoglio B, Veronese S, Siena S, Sartore-Bianchi A, et al. The molecular landscape of colorectal cancer cell lines unveils clinically actionable kinase targets. Nat. Commun. 2015;6:7002. doi: 10.1038/ncomms8002. [DOI] [PubMed] [Google Scholar]
- 8.Brattain MG, Fine WD, Khaled FM, Thompson J, Brattain DE. Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res. 1981;415:1751–1756. [PubMed] [Google Scholar]
- 9.von Kleist S, Chany E, Burtin P, King M, Fogh J. Immunohistology of the antigenic pattern of a continuous cell line from a human colon tumor. J.Natl.Cancer Inst. 1975;553:555–560. doi: 10.1093/jnci/55.3.555. [DOI] [PubMed] [Google Scholar]
- 10.Leibovitz A, Stinson JC, McCombs WB, III, McCoy CE, Mazur KC, Mabry ND. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976;3612:4562–4569. [PubMed] [Google Scholar]
- 11.Drewinko B, Romsdahl MM, Yang LY, Ahearn MJ, Trujillo JM. Establishment of a human carcinoembryonic antigen-producing colon adenocarcinoma cell line. Cancer Res. 1976;362:467–475. [PubMed] [Google Scholar]
- 12.Lange F, Franz B, Maletzki C, Linnebacher M, Huhns M, Jaster R. Biological and molecular effects of small molecule kinase inhibitors on low-passage human colorectal cancer cell lines. Biomed.Res.Int. 2014;2014:568693. doi: 10.1155/2014/568693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowehl RA, Burke S, Bialkowska AB, Pettet DW, III, Rowehl L, Li E, Antoniou E, Zhang Y, Bergamaschi R, Shroyer KR, Ojima I, Botchkina GI. Establishment of highly tumorigenic human colorectal cancer cell line (CR4) with properties of putative cancer stem cells. PLoS. One. 2014;96:e99091. doi: 10.1371/journal.pone.0099091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maletzki C, Stier S, Gruenert U, Gock M, Ostwald C, Prall F, Linnebacher M. Establishment, characterization and chemosensitivity of three mismatch repair deficient cell lines from sporadic and inherited colorectal carcinomas. PLoS. One. 2012;712:e52485. doi: 10.1371/journal.pone.0052485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maletzki C, Gock M, Randow M, Klar E, Huehns M, Prall F, Linnebacher M. Establishment and characterization of cell lines from chromosomal instable colorectal cancer. World J. Gastroenterol. 7-1-2015;211:164–176. doi: 10.3748/wjg.v21.i1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grasso S, Martinez-Lacaci I, Barbera VM, Castillejo A, Soto JL, Gallego-Plazas J, Lopez-Riquelme N, Garcia-Morales P, Mata-Balaguer T, Ferragut JA, Saceda M. HGUE-C-1 is an atypical and novel colon carcinoma cell line. BMC Cancer. 2015;15:240. doi: 10.1186/s12885-015-1183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castle JC, Loewer M, Boegel S, de GJ, Bender C, Tadmor AD, Boisguerin V, Bukur T, Sorn P, Paret C, Diken M, Kreiter S, Tureci O, Sahin U. Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genomics. 2014;15:190. doi: 10.1186/1471-2164-15-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao GH, Liu HM, Li BW, Hao JJ, Yang YL, Wang MR, Wang XH, Wang J, Jin HJ, Du L, Chen Q. Establishment of a human colorectal cancer cell line P6C with stem cell properties and resistance to chemotherapeutic drugs. Acta Pharmacol. Sin. 2013;346:793–804. doi: 10.1038/aps.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JS, Kim L, Kim CH, Bang BW, Lee DH, Jeong S, Shin YW, Kim HG. Synchronous large-cell neuroendocrine carcinoma and adenocarcinoma of the colon. Gut Liver. 2010;41:122–125. doi: 10.5009/gnl.2010.4.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gravante G, Markiewicz D, Madeddu F, Giordano P. Colonic large-cell neuroendocrine tumours. Can.J.Surg. 2009;523:E49–E51. [PMC free article] [PubMed] [Google Scholar]
- 21.de Miranda NF, van Dinther M, van den Akker BE, van Wezel T, Ten Dijke P, Morreau H. Transforming Growth Factor beta Signaling in Colorectal Cancer Cells with Microsatellite Instability Despite Biallelic Mutations in TGFBR2. Gastroenterology. 28-2-2015;15:301–307. doi: 10.1053/j.gastro.2015.02.052. [DOI] [PubMed] [Google Scholar]
- 22.Shima K, Morikawa T, Baba Y, Nosho K, Suzuki M, Yamauchi M, Hayashi M, Giovannucci E, Fuchs CS, Ogino S. MGMT promoter methylation, loss of expression and prognosis in 855 colorectal cancers. Cancer Causes Control. 2011;222:301–309. doi: 10.1007/s10552-010-9698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang W, Wang PG, Zhan Y, Zhang D. Prognostic value of p16 promoter hypermethylation in colorectal cancer: a meta-analysis. Cancer Invest. 2014;322:43–52. doi: 10.3109/07357907.2013.861476. [DOI] [PubMed] [Google Scholar]
- 24.Paz MF, Fraga MF, Avila S, Guo M, Pollan M, Herman JG, Esteller M. A systematic profile of DNA methylation in human cancer cell lines. Cancer Res. 1-3-2003;635:1114–1121. [PubMed] [Google Scholar]
- 25.Suter CM, Norrie M, Ku SL, Cheong KF, Tomlinson I, Ward RL. CpG island methylation is a common finding in colorectal cancer cell lines. Br.J.Cancer. 10-2-2003;883:413–419. doi: 10.1038/sj.bjc.6600699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin G, Kang TW, Yang S, Baek SJ, Jeong YS, Kim SY. GENT: gene expression database of normal and tumor tissues. Cancer Inform. 2011;10:149–157. doi: 10.4137/CIN.S7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janakiraman M, Vakiani E, Zeng Z, Pratilas CA, Taylor BS, Chitale D, Halilovic E, Wilson M, Huberman K, Ricarte Filho JC, Persaud Y, Levine DA, Fagin JA, et al. Genomic and biological characterization of exon 4 KRAS mutations in human cancer. Cancer Res. 15-7-2010;7014:5901–5911. doi: 10.1158/0008-5472.CAN-10-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, Monsey J, Goel N, Aronson AB, Li S, Ma CX, Ding L, Mardis ER, Ellis MJ. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;32:224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 19-7-2012;4877407:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corver WE, Ruano D, Weijers K, den Hartog WC, van Nieuwenhuizen MP, de Miranda N, van Eijk R, Middeldorp A, Jordanova ES, Oosting J, Kapiteijn E, Hovens G, Smit J, et al. Genome haploidisation with chromosome 7 retention in oncocytic follicular thyroid carcinoma. PLoS. One. 2012;76:e38287. doi: 10.1371/journal.pone.0038287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruin SC, Klijn C, Liefers GJ, Braaf LM, Joosse SA, van Beers EH, Verwaal VJ, Morreau H, Wessels LF, van Velthuysen ML, Tollenaar RA, Van't Veer LJ. Specific genomic aberrations in primary colorectal cancer are associated with liver metastases. BMC Cancer. 2010;10:662. doi: 10.1186/1471-2407-10-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.TCGA Comprehensive molecular characterization of human colon and rectal cancer. Nature. 19-7-2012;4877407:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Kuppeveld FJ, van der Logt JT, Angulo AF, van Zoest MJ, Quint WG, Niesters HG, Galama JM, Melchers WJ. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl.Environ.Microbiol. 1992;588:2606–2615. doi: 10.1128/aem.58.8.2606-2615.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Roon EH, Boot A, Dihal AA, Ernst RF, van Wezel T, Morreau H, Boer JM. BRAF mutation-specific promoter methylation of FOX genes in colorectal cancer. Clin. Epigenetics. 2013;51:2–3. doi: 10.1186/1868-7083-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Eijk R, Licht J, Schrumpf M, Talebian YM, Ruano D, Forte GI, Nederlof PM, Veselic M, Rabe KF, Annema JT, Smit V, Morreau H, van Wezel T. Rapid KRAS, EGFR, BRAF and PIK3CA mutation analysis of fine needle aspirates from non-small-cell lung cancer using allele-specific qPCR. PLoS. One. 2011;63:e17791. doi: 10.1371/journal.pone.0017791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venkatraman ES, Olshen AB. A faster circular binary segmentation algorithm for the analysis of array CGH data. Bioinformatics. 15-3-2007;236:657–663. doi: 10.1093/bioinformatics/btl646. [DOI] [PubMed] [Google Scholar]
- 37.Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2. 0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011;124:R41. doi: 10.1186/gb-2011-12-4-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 20-1-2015;7:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


