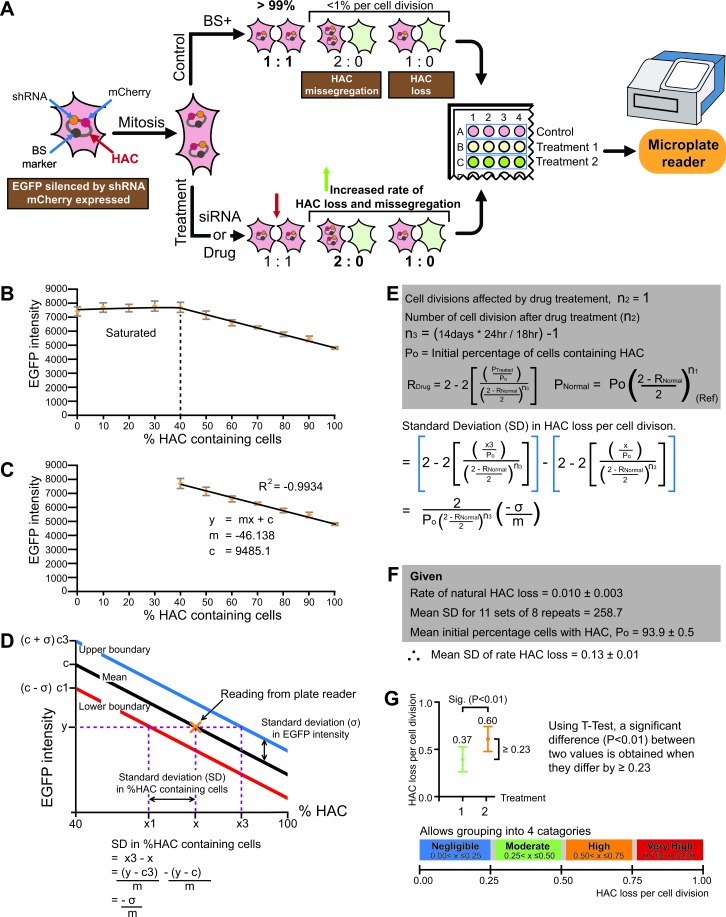
Figure 4.
A. Scheme of a high throughput assay for measuring chromosome instability (CIN). During S-phase the HAC is duplicated in the cell as other host chromosomes. Under normal growth conditions (control), after cell division the majority of cells in population contain the HAC (1:1 segregation). After drug or siRNA treatment, one copy of HAC can be lost during cell division (1:0 segregation) or two copies of HAC can mis-segregate (2:0 segregation). The cells that inherited the HAC are fluorescent red while cells that lost it are fluorescent green. For each treatment, the average fluorescence of each cell sample may be measured using a multi-well plate reader. Thus, the genes involved in chromosome transmission or drugs affecting chromosome stability may be identified. B. An example of a titration curve between EGFP intensity against cell populations of varying percentage of HAC-containing (not green) and HAC-less (green) cells obtained from a fluorescence microtiter plate reader. C. Construction of a linear curve between a cell population's EGFP intensity against the percentage of HAC-containing cells. D. The relationship between the standard deviation of a cell population's EGFP intensity and it's percentage of HAC-containing cells. E. The relationship between the standard deviation of HAC-containing cells and the ‘rate of HAC loss per cell division'. F. The estimated standard deviation in the ‘rate of HAC loss' using data derived from a fluorescence plate reader and known rates of natural HAC loss. G. The minimum difference between two values of ‘HAC loss' needed to be significant (P < 0.01) was calculated using a T-test and the standard deviations derived above. The results indicate that plate reader is only able to discriminate drugs into broad categories.