Fig 5. Mutation of hOSTβ (LL/AA) results in predicted alterations to protein structure and hydrophobicity.
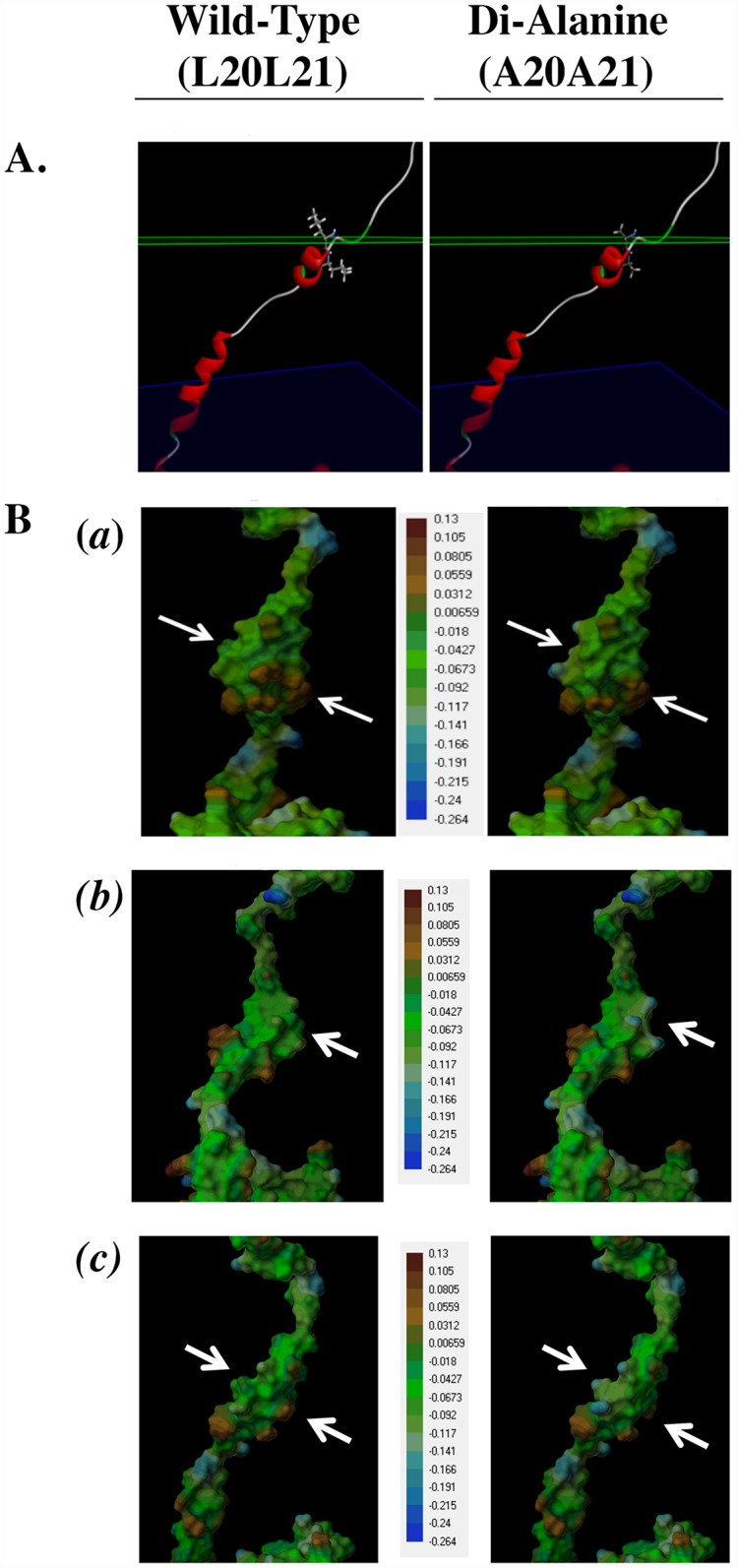
(A) The location of the two leucine residues near one edge of the membrane in the wild-type OSTβ (left panel) is predicted to be in a transmembrane region of the protein near the extracellular edge of the hydrophobic region of the membrane (green plane) in positions that are accessible for interaction with OSTα. Mutation of these two residues to Ala (right panel) reduces the size of the side chains at these positions. (B) Molecular surface representations (a, b, c) of the transmembrane region of wild-type OSTβ (left panel) and the LL/AA mutant (right panel) colored based on a lipophilic potential gradient with brown designated as most hydrophobic and blue as least hydrophobic. The mutation substantially decreases the lipophilic potential in this area of the surface (arrows) in addition to altering the size and volume of this region of the protein.
