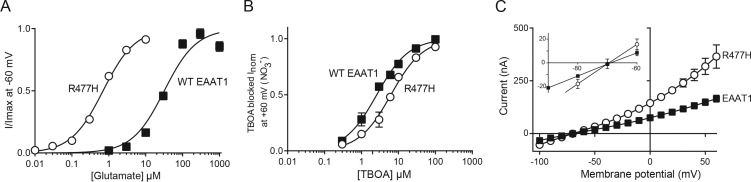
Figure 4.
The substrate-binding site is unperturbed in R477H at pH 5.5. (A) Steady-state currents at 60 mV in the presence of increasing concentrations of the competitive blocker TBOA were measured in WT EAAT1 and the R477H mutant transporter at pH 5.5. These currents were then subtracted from currents in the absence of TBOA to measure the TBOA-dependent blockage of the Na+-activated anion conductance. Currents were normalized to the maximal current blocked by TBOA for each cell. (B) l-Glutamate concentration responses for WT EAAT1 (closed squares) and R477H (open circles) at pH 5.5 reproduced from Fig. 2 are provided for comparison with [TBOA] concentration responses. (C) Current-voltage relationships for WT EAAT1 (closed squares) and R477H (open circles) as blocked by 30 µM TBOA at pH 5.5. Data shown represent the mean ± SEM (n ≥ 4). Where error bars cannot be seen, they lie within the symbol.