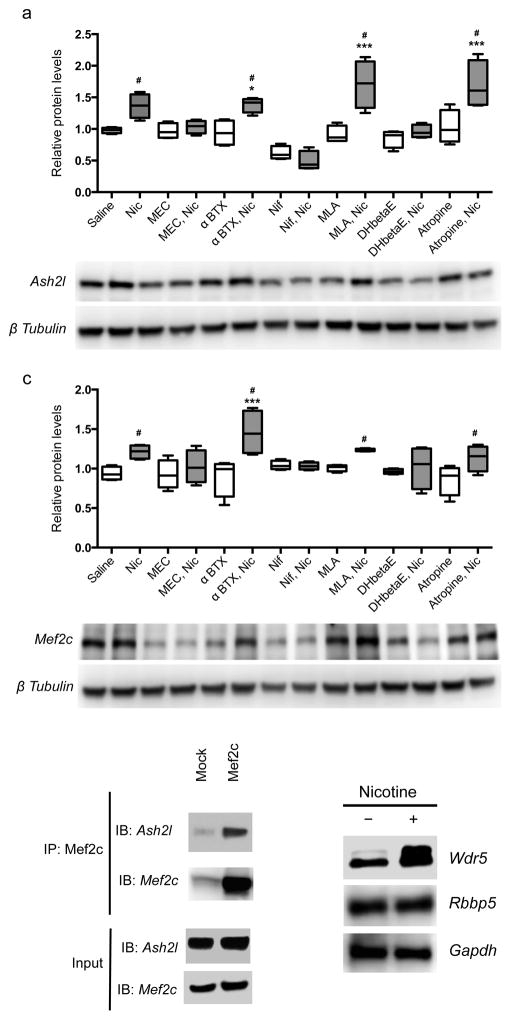
Figure 4. The Ash2l/Mef2c complex is regulated by nAChR activity.
(a, b) Nicotine treatment of neural progenitor cells in culture significantly elevated Ash2l (a) and Mef2c (b) protein levels (F(13,42) = 12.540, p = 0.000001 (Saline vs Nic: p = 0.009350; α-BTX vs α-BTX+Nic: p = 0.003186; MLA vs MLA+Nic: p = 0.000001; Atropine vs Atropine+Nic: p = 0.000030) and F(13,42) = 3.749, p = 0.000551 (Saline vs Nic: p = 0.023076; α-BTX vs α-BTX+Nic: p = 0.010348; MLA vs MLA+Nic: p = 0.05; Atropine vs Atropine+Nic: p = 0.023488), respectively, as measured by one-way ANOVA followed by LSD test for multiple comparisons). This was reversed by the broad spectrum nAChR antagonist mecamylamine (MEC) and the more selective heteromeric nAChR antagonist dihydro-β-erythroidine (DHβE). The selective α7 nAChR antagonists α-bungarotoxin (btx) and methyllyccaconitine (MLA) had no effect on their own and did not alter the effects of nicotine (Nic). The voltage-gated calcium channel blocker nifedipine (Nif) decreased levels of Ash2l and Mef2c at baseline and abolished the ability of nicotine to increase their expression. (c) Ash2l and Mef2c can be co-immunoprecipitated in a complex from neural progenitor cells (replicated twice). (d) Wdr 5 protein levels are increased by nicotine treatment. n = 4 replicates per condition for all neural progenitor experiments. Original Western blots presented in supplementary Fig. 7. Whiskers represent minimum to maximum value of data distribution.