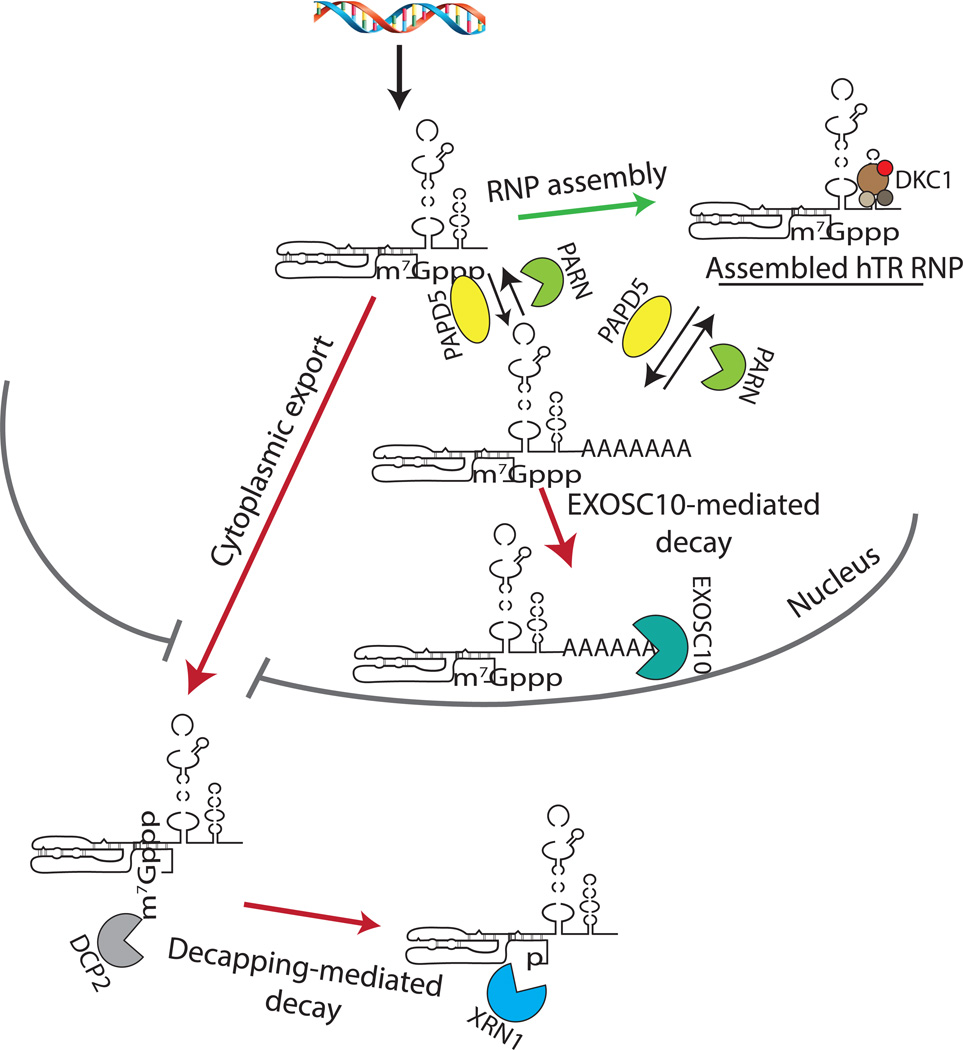
Figure 2. Competition Between hTR RNP Assembly and Quality Control.
Under normal conditions, hTR is bound by dyskerin and other H/ACA proteins to form the hTR RNP. In DC, unassembled hTR is degraded by EXOSC10 in the nucleus, and by DCP2/XRN1 in the cytoplasm. DCP2 removes the m7Gpp cap structure from the 5’ end of hTR, exposing the monophosphate to allow XRN1-mediated exonucleolytic digestion. Loss of PARN also leads to degradation of hTR by EXOSC10, which is aided by PAPD5-mediated oligoadenylation of hTR 3’ end, where it can destabilize both unbound and assembled hTR molecules.