Abstract
Except a small population of primary afferent neurons for sensing cold to generate the sensations of innocuous and noxious cold, it is generally believed that cold temperatures suppress the excitability of other primary afferent neurons that are not for cold-sensing. These not-for-cold-sensing neurons include the majority of non-nociceptive and nociceptive afferent neurons. In the present study we have found that not-for-cold-sensing neurons of rat trigeminal ganglia (TG) change their excitability in several ways at cooling temperatures. In nearly 70% of not-for-cold-sensing TG neurons, the cooling temperature of 15°C increases their membrane excitability. We regard these neurons as cold-active neurons. For the remaining 30% of not-for-cold-sensing TG neurons, the cooling temperature of 15°C either has no effect (regarded as cold-ineffective neurons) or suppress (regarded as cold-suppressive neurons) their membrane excitability. For cold-active neurons, the cold temperature of 15°C increases their excitability as is evidenced by the increases in action potential (AP) firing numbers and/or reduction of AP rheobase when these neurons are depolarized electrically. The cold temperature of 15°C significantly inhibits M-currents and increases membrane input resistance of cold-active neurons. Retigabine, an M-current activator, abolishes the effect of cold temperatures on AP firing but not the effect of cold temperature on AP rheobase levels. The inhibition of M-currents and the increases of membrane input resistance are likely two mechanisms by which cooling temperatures increase the excitability of not-for-cold-sensing TG neurons.
Keywords: cold, pain, KCNQ channels, retigabine, trigeminal ganglion neurons, M-currents
Introduction
Cold temperatures can excite a subpopulation of trigeminal and spinal afferent fibers to generate sensory impulses and produce the sensations of pleasant cooling and noxious cold (Morin and Bushnell, 1998). These afferents are regarded as cold-sensing, cold-sensitive, or cold-responsive afferent neurons (Reid et al., 2002, Babes et al., 2004, McKemy, 2005). In addition to their physiological functions in sensing cold temperatures, these afferents play a role in pathological pain such as cold allodynia and hyperalgesia (McKemy, 2005, Xing et al., 2007). Studies have demonstrated that TRPM8 channels are the principal cold sensor in cold-sensing afferent neurons; cold temperatures open TRPM8 channels to cause membrane depolarization and AP firing in the cold-sensing afferent neurons (McKemy et al., 2002, Peier et al., 2002). TRPA1 channels have also been reported to be cold transducers but controversial results have been obtained from different laboratories (Story et al., 2003, Jordt et al., 2004). A recent study has shown that TRPM8-expressing sensory neurons form a sensory-labeled line that defines the cellular basis for cold and cold pain (Knowlton et al., 2013). However, the transduction of cold stimuli into sensory impulses in this labeled line may be significantly influenced by a number of channels including A-type K+ channels (Sarria et al., 2012), Kv1 channels (Madrid et al., 2009), K2P channels (Reid and Flonta, 2001, Enyedi and Czirjak, 2010) and perhaps other ion channels that are involved in setting the excitability of the cold-sensing neurons. Cold-sensing neurons represent a small subpopulation of somatosensory neurons (Xing et al., 2006, Xing et al., 2007). Most primary afferent neurons are not for cold-sensing, and these not-for-cold-sensing neurons do not transduce cold stimuli into afferent impulses for cold sensations. It has been generally believed that, except for cold-sensing neurons, cold temperatures suppress the excitability of other neurons.
We have previously shown in cultured DRG neurons that cold temperatures suppress the excitability of some DRG neurons but can increase the excitability of other DRG neurons (Sarria et al., 2012). Cultured DRG neurons whose excitability is suppressed by cold temperatures are found to be those that predominantly express TTX-sensitive voltage-gated sodium channels (VGSCs) (Sarria et al., 2012). Studies have shown that cold temperatures strongly inhibit TTX-sensitive VGSCs due to voltage-dependent slow inactivation of these VGSCs at cooling temperatures (Zimmermann et al., 2007, Sarria et al., 2012), which accounts for the suppression of the excitability of non-nociceptive afferent neurons by cold temperatures (Zimmermann et al., 2007, Sarria et al., 2012). In contrast to non-nociceptive primary afferent neurons, nociceptive neurons express high amounts of VGSCs that are TTX-resistant. TTX-resistant VGSCs show little voltage-dependent slow inactivation at cooling temperatures, a property essential for nociceptive neurons to detect noxious stimuli at cold temperatures (Zimmermann et al., 2007, Sarria et al., 2012). Our previous study in cultured DRG neurons indicates that TTX-resistant VGSCs are also required for DRG neurons to display enhanced excitability at cold temperatures (Sarria et al., 2012).
Based on our previous study with cultured DRG neurons, one mechanism underlying the enhancement of nociceptive-like neuron excitability is the inhibition of A-type K+ channels (IA) by cold temperatures (Sarria et al., 2012). IA currents in nociceptive neurons serve as a brake to oppose membrane depolarization and limit action potential (AP) firing. IA currents in nociceptive neurons are inhibited significantly by cooling temperatures, which releases the brake to facilitate AP firing in nociceptive neurons. In nociceptive neurons, IA channels are mainly Kv1.4, Kv4.1 and Kv4.3 channels (Rasband et al., 2001, Phuket and Covarrubias, 2009). They belong to the low threshold voltage-gated K+ channels (KLT) that start to open at low voltages near resting membrane potentials (Johnston et al., 2010). In addition to IA channels, M-type K+ channel (M-channel) is also a major type of KLT channels. M-channels in DRG neurons are mainly KCNQ2/3 heteromeric channels (Passmore et al., 2003, Rose et al., 2011, Zheng et al., 2013) and both KCNQ2 and KCNQ3 subunits are expressed in nociceptive DRG neurons (Passmore et al., 2003, Rose et al., 2011, Zheng et al., 2013). M-channel expression is down-regulated after nerve injury and bone cancer in animals (Rose et al., 2011, Zheng et al., 2013), which is associated with the increased excitability in nociceptive DRG neurons (Duan et al., 2012, Zheng et al., 2013). More recently we have shown that KCNQ channels in nociceptive cold-sensing TG neurons are therapeutic targets for treating orofacial cold hyperalgesia, and this study further highlights the significant role of M-channels in controlling nociceptive TG neuron excitability (Abd-Elsayed et al., 2015). In addition to KLT channels, the excitability of primary afferents is also affected by K2P channels (Yamamoto et al., 2009, Enyedi and Czirjak, 2010). K2P channels are leak K+ channels that include TWIK, TREK, TASK, TALK, THIK, and TRESK. These leak K+ channels play an important role in setting resting membrane potentials and membrane input resistances (Enyedi and Czirjak, 2010). In the present study, we use whole-mount TG preparation to examine the effects and underlying mechanism of cold temperatures on the excitability of TG neurons that are not for cold-sensing.
Materials and Methods
Tissue preparation
Sprague Dawley rats aged 5-7 weeks were used. Animal care and use conformed to NIH guidelines for care and use of experimental animals. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Animals were sacrificed and trigeminal ganglions (TGs) were dissected out from the animals. Under a dissection microscope, connective tissues on the surface of TGs were removed carefully by a pair of forceps. The TGs were then fixed in a recording chamber with a tissue anchor and submerged in a Krebs solution that contained (in mM): 117 NaCl, 3.5 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3 and 11 glucose; the solution was saturated with 95% O2 and 5% CO2, had pH of 7.3 and osmolarity of 325 mOsm. Unless otherwise indicated, the temperature of the bath solution was maintained at 24°C. The recording chamber was mounted on the stage of an Olympus IX50 microscope that was equipped with IR-DIC and fluorescent imaging systems. The TGs were exposed to 0.05% dispase II plus 0.01% collagenase in the Krebs solution for 8-15 min, and the enzymes were then washed off with the Krebs solution. Under a 40X objective and DIC-infrared optical system, individual TG neurons were visualized for patch-clamp recordings.
Patch-clamp recordings
Patch-clamp recordings were made from small-sized TG neurons (diameter from 15 to 30 μm) situated in whole-mount TGs. In brief, recording electrodes were fabricated by using a P-97 Brown-Flaming Micropipette Puller. The resistance of each electrode was ~6 MΩ after filling with recording internal solution. Recording internal solution was a K+-based solution containing (in mM): 135 K-gluconate, 5 KCl, 0.5 CaCl2, 2 MgCl2, 5 EGTA, 5 HEPES, 5 Na2ATP and 0.5 GTP-TRIS salt; the pH of the solution was adjusted to 7.3 with KOH. To make a membrane seal with a targeted TG neuron in the whole-mount TG preparation, a positive pressure was delivered into a patch-clamp recording electrode before and during approaching the cell. The positive pressure was generated by compressing ~5 ml air in a 50-ml syringe that was connected to the recording electrode. The electrode tip was then lowered until it penetrated the satellite cell that wrapped on the targeted TG neuron. The positive pressure in the recording electrode was then reduced and the position of the electrode tip adjusted to allow it to directly contact the membrane of the targeted TG neuron. Once the recording electrode touched TG neuron membranes, the positive pressure was gradually reduced and a negative pressure was applied through the syringe until gigaohm seal was formed (2 to 4 GΩ) between the recording electrode and the TG neuron membranes. The whole-cell recording made from a TG neuron in our whole-mount TG preparation was normally stable for at least 2 hours and no significant changes in basic electrophysiological parameters were observed. Signals were recorded and amplified using an Axopatch 200B amplifier, filtered at 2 kHz and sampled at 5 kHz using pCLAMP 10 software (Molecular Devices).
Testing electrophysiological properties and neuronal excitability
To determine membrane and AP properties of recorded TG neurons, under the whole-cell current clamp mode, step current pulses were injected into cells through patch-clamp electrodes. The step currents were applied from −50 pA to 1050 pA in an increment of 50 pA per step and the duration of each pulse was 250 ms. For voltage-clamp recordings, TG neurons were held at −75 mV unless otherwise indicated. To determine voltage-gated Na+ currents, whole-cell inward currents were evoked by a series of voltage steps from a holding potential of −75 mV, and voltage steps were ranging from −90 to 30 mV with a 10 mV increment each step and the duration of each step was 250 ms. Isolation of sodium currents was not performed in this study because we intended to keep the preparation in a relatively normal condition for multiple recordings. To determine M-currents in TG neurons, deactivating tail currents were obtained following the voltage step of −20 mV as described in our previous study (Abd-Elsayed et al., 2015). In all electrophysiology experiments, unless otherwise indicated, membrane voltages mentioned in the texts have been corrected for calculated junction potentials. Most recordings were performed at both 24°C and 15°C. In one set of experiments, recordings were performed in a series of temperatures at 35, 30, 25, 20, 15, 10 and 5°C.
Application of cooling temperatures and drugs
The temperatures of bath solutions were controlled by a Peltier cooling device (Model TCM-1, Warner Instrument, CT, USA), which were delivered to patched cells from a short tube (0.2 cm L, 500 μm ID) with the outlet 2 mm away from the recorded cells. The temperatures at the recording sites were continuously recorded with a thermal probe that attached to the controller of the Peltier cooling device. For voltage- and current-clamp experiments, a targeted temperature, e.g. 15°C, was reached first and then voltage and/or current protocols were applied to determine changes in currents and membrane properties, respectively. Testing drugs include linopirdine (10 μM) and retigabine (10 μM). They were delivered with the bath solution in the same tube and were tested at 24°C or 15°C.
Data Analysis
Whole-cell recordings from voltage- and current-clamp experiments were analyzed using Clampfit 10 software. The measurements of membrane and AP parameters were performed in the same manner as a previous study by us (Sarria et al 2012). Data are reported as mean ± SEM. Statistical significance (*p < 0.05, **p < 0.01, and ***p < 0.001) was assessed by Student’s t test, Fisher’s exact test, or one-way ANOVA with Bonferroni post hoc test.
Results
Different from most previous studies using either acutely dissociated or cultured TG neurons, neurons in our whole-mount TG preparation better represent cells in an intact condition. All recordings were made from the V2 region of TG (Figure 1A&B) where neurons mainly innervate the orofacial area. We injected currents via recording electrodes to depolarize TG neuron membranes and elicit AP firing, and determined TG neuron excitability at the control condition of 24°C and the cooling temperature of 15°C (Figure 1C-L). AP numbers and rheobase levels were used as two main parameters of TG neuron excitability. Neurons included in the present study were those that neither displayed detectable inward currents (under voltage-clamp mode) nor fire APs (under current-clamp mode) when the cooling temperature ramp of 24 °C to 5°C was applied to these neurons. We regarded these neurons as not-for-cold-sensing neurons in the present study.
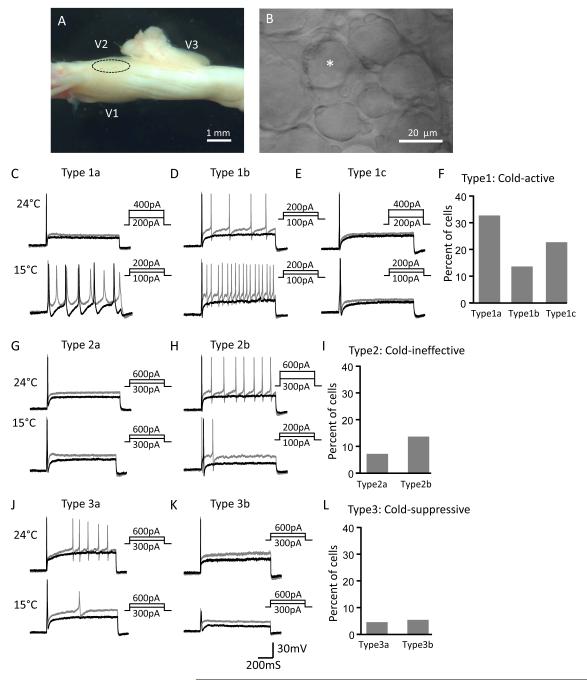
Figure 1. Multiple effects on the excitability of not-for-cold-sensing trigeminal ganglion neurons by the cold temperature of 15°C.
A) Image shows a whole-mount trigeminal ganglion (TG) preparation for patch-clamp recordings from TG neurons. A circle indicates the V2 region of the TG. B) Image shows some TG neurons in the V2 region of the TG viewed under a 40x objective. The asterisk indicates a small-sized TG neuron being recorded. C) Two sets of sample traces show action potential (AP) firing in a TG neuron at 24°C (top panel) and 15°C (bottom panel). The cell (type 1a) shows an increase in AP firing numbers and a decrease in rheobase at 15°C. AP firing was elicited by the injections of depolarizing currents. Black and gray traces in C-L are membrane responses at the rheobase and 2x rheobase levels, respectively. D) A different cell (type 1b) shows an increase in AP firing numbers without a change of rheobase at 15°C. E) A cell (type 1c) shows no change in AP firing numbers but a decrease in rheobase at 15°C. F) Percent of each cold-active cell subtype represented in C, D and E. The percentage is based on the total TG neurons tested, which include all type 1 cells and also two other cell types described below. G) A cell (type 2a) shows neither a change in AP firing numbers nor a change in rheobase at 15°C. H) A cell (type 2b) shows a decrease in AP firing numbers and a decrease in rheobase at 15°C. I) Percent of each cold-ineffective cell subtype represented by G and H in the total TG neurons tested. J) A cell (type 3a) shows a decrease in AP firing numbers and no change in rheobase at 15°C. K) A cell (type 3b) shows the failure of AP at 15°C. L) Percent of each cold-suppressive cell subtype represented by J and K in the total TG neurons tested.
A total of 112 not-for-cold-sensing neurons were tested for their excitability at 24°C and 15°C. On the basis of the changes in AP firing numbers and rheobase levels in response to temperature drop from 24°C to 15°C, the not-for-cold-sensing TG neurons could be classified into three major types, cold-active type (type 1) in which the neurons became more excitable at the cold temperature of 15°C (n = 77, Figure 1C-F), cold-ineffective type (type 2) in which the excitability of the neurons were not significantly affected at 15°C (n = 23, Figure 1G-I), and cold-suppressive type (type 3) in which the excitability of the neurons were significantly reduced at 15°C (n = 12, Figure 1J-L). Cold-active neurons can be further classified into three subtypes, type 1a, type 1b, and type 1c. Type 1a cells showed increases in AP firing numbers and decreases in rheobase levels at 15°C (Figure 1C), and they (n = 37) accounted for 33% of the total neurons tested (Figure 1F). Type 1b cells displayed increases in AP firing numbers but no change in the rheobase levels at 15°C (Figure 1D), and these neurons (n = 15) accounted for 14% of the total neurons recorded (Figure 1F). Type 1c cells had the reduced rheobase levels but no change in AP firing numbers (Figure 1E), and they (n = 25) accounted for 23% of the total neurons recorded (Figure 1F). Together, type 1 neurons (n = 77) accounted for 70% of the total neurons recorded.
Cold-ineffective TG neurons could be classified into two subtypes, type 2a and type 2b. Type 2a cells showed neither a change of AP numbers nor a change of rheobase levels at 15°C (Figure 1G), and they (n = 8) accounted for 7% of total neurons (Figure 1I). Type 2b cells displayed decreased AP firing numbers and decreased rheobase levels at 15°C (Figure 1H), and they (n = 15) accounted for 14% of the total neurons (Figure 1I). We placed these cells in the cold-ineffective subgroup because these cells showed decreases in excitability when judged by AP firing numbers but increases in excitability when judged by rheobase levels. Cold-suppressive TG neurons had two subtypes, type 3a and type 3b. Type 3a neurons showed decreases in AP firing numbers but no change in the rheobase levels (Figure 1J) at 15°C, and they (n = 5) accounted for 4% of the total neurons (Figure 1L). Type 3b neurons failed to fire APs at 15°C (Figure 1K), and they (n = 7) accounted for 5% of the total neurons (Figure 1L).
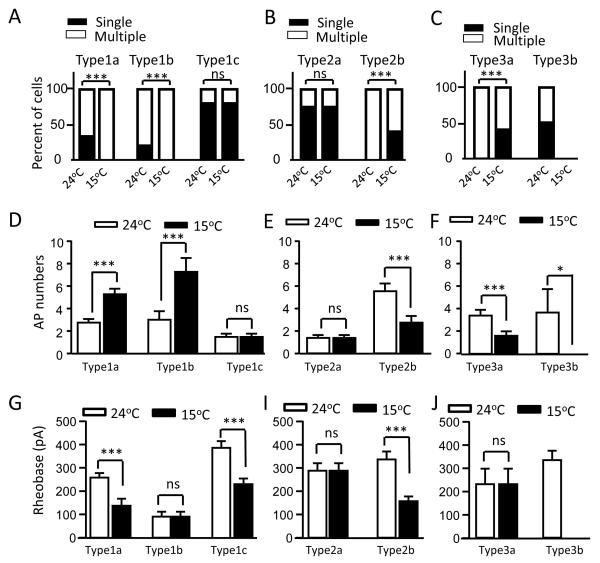
We examined effects of cooling temperatures on AP firing patterns. At 24°C, not-for-cold-sensing neurons have two main AP firing patterns, phasic firing (e.g. Figure 1E) and multiple-firing (e.g. Figure 1D). For a phasic firing neuron, only a single AP could be elicited by threshold and above-threshold depolarizations. For a multiple firing neuron, two or more APs could be elicited by threshold and/or above-threshold depolarizations. Multiple firing neurons could be further classified into several subgroups such as tonic, adapting and irregular firing patterns (Sarria et al., 2012), but for simplicity we did not make such sub-classification in the present study. At 24°C, the majority of type 1a cells (66%, 24/37) and type 1b cells (80%, 12/15) fired multiple APs (Figure 2A), and 33% (13/37) of type 1a cells and 20% (3/15) of type 1b neurons showed phasic firing (Figure 2A). When these cells were tested at 15°C, all of them increased their AP firing numbers and all of the phasic firing cells switched to multiple-firing pattern (Figure 2A). Most of type 1c cells (80%, 20/25) were phasic firing cells at 24°C and they did not change their firing pattern at 15°C (Figure 2A). For type 2a neurons, most of them (75%, 6/8) showed phasic firing at 24°C and there was no change in firing pattern at 15°C (Figure 2B). All type 2b neurons (100%, 15/15) fired multiple APs at 24°C and some of them (40%, 6/15) became phasic firing at 15°C (Figure 2B). Type 3a neurons also all (100%, 5/5) fired multiple APs at 24°C and some of them (40%, 2/5) became phasic firing at 15°C (Figure 2C). For type 3b neurons, some of them showed phasic firing (50%, 3/6) while others displayed multiple firing pattern (50%, 3/6) at 24°C and they all failed to fire APs at 15°C (Figure 2C, n = 6). Figure 2D-F shows overall changes of AP firing numbers at 24°C and 15°C for each cell type. With temperature drop from 24°C to 15°C, AP firing numbers were increased from 2.75 ± 0.33 to 5.28 ± 0.49 (n = 36, P < 0.001) for type 1a cell, from 3.00 ± 0.77 to 7.27 ± 1.24 (n = 16, P < 0.001) for type 1b, and no change for type 1c (n = 25) (Figure 2D). For type 2 (Figure 2E) and type 3 (Figure 2F) cells, with temperature cooled down from 24°C to 15°C, AP fire numbers were not changed for type 2a cells, decreased from 5.53 ± 0.69 to 2.73 ± 0.60 (n = 15, P < 0.001) for type 2b, decreased from 3.40 ± 0.51 to 1.60 ± 0.40 (n = 5, P < 0.001) for type 3a cells. Type 3b had AP numbers of 3.67 ± 2.09 at 24°C and they all (n = 6, P < 0.05) failed to fire APs at 15°C. With temperature drop from 24°C to 15°C, rheobase levels were reduced from 258.0 ± 19.9 pA to 137.5 ± 30.2 pA (n = 37, P < 0.001) for type 1a cells, from 386.0 ± 29.4 pA to 230 ± 24.5 pA (n = 25, P < 0.001) for type 1c, and from 336.7 ± 33.6 pA to 156.7 ± 20.6 pA (n = 15, P < 0.001) for type 2b cells (Figure 2G). There was no significant change in rheobase levels for other cell types with temperature drop from 24°C to 15°C (Figure 2G-I).
Figure 2. Action potential firing patterns, numbers, and rheobase levels of not-for-cold-sensing neurons at 24°C and 15°C.
A) Percept of the cells showing multiple AP firing and phasic (single) AP firing patterns in each subtype of type 1 cells. B) Percept of the cells showing multiple firing and phasic firing in each subtype of type 2 cells. C) Percept of the cells showing multiple firing and phasic firing in each subtype of type 3 cells. In A to C, cell subtypes are indicated above bars and temperatures under bars. Open bars and solid bars represent multiple and phasic AP firing cells, respectively. D) AP firing numbers of type 1a (n = 37), type 1b (n = 15) and type 1c cells (n = 25) at 24°C (open bars) and 15°C (closed bars). E) AP firing numbers of type 2a (n = 8) and type 2b cells (n = 15) at 24°C (open bars) and 15°C (closed bars). F) AP firing numbers of type 3a (n = 6) and type 3b (n = 6) at 24°C and 15°C. In D to F, AP firing numbers were obtained at 2x rheobase levels. G) Rheobase levels of type 1a (n = 37), type 1b (n = 15) and type 1c cells (n = 25) at 24°C and 15°C. H) Rheobase levels of type 2a (n = 8) and type 2b cells (n = 15) at 24°C and 15°C. I) Rheobase levels of type 3a (n = 6) and type 3b (n = 6) at 24°C and 15°C. Data represent Mean ± SEM, ** P < 0.01, *** P < 0.001; ns, no significant difference.
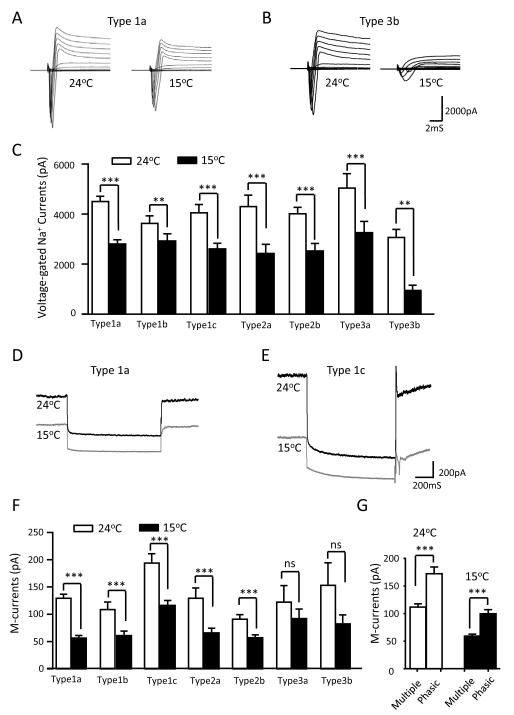
We examined voltage-gated Na+ currents at 24°C and 15°C for each cell type (Figure 3A-C). Although the cold temperature of 15°C inhibited voltage-gated Na+ currents in each type of cells, substantial amount of voltage-gated Na+ currents remained (peak amplitude > 2500 pA) at 15°C for all cell types except type 3b neurons. For example, peak amplitude of voltage-gated Na+ currents in type 1a cells was 4539 ± 208 pA at 24°C and 63% (2861 ± 161 pA, n = 36) of the currents remained at 15°C (Figure 3A&C). However, voltage-gated Na+ currents in type 3b neurons were substantially inhibited, and their peak amplitude was inhibited by about 60% from 3064 ± 322 pA at 24°C to 944 ± 207 pA (n = 6, P < 0.01) at 15°C (Figure 3B&C). The profound inhibition of voltage-gated Na+ currents in type 3b cells was consistent with the failure of AP firing for these cells at 15°C.
Figure 3. Voltage-gated Na+ currents and M-currents in not-for-cold-sensing neurons 24°C and 15°C.
A) Sample traces of peak voltage-gated Na+ currents of a type 1a cell at 24°C and 15°C. B) Sample traces of peak voltage-gated Na+ currents of a type 3b cell at 24°C and 15°C. C) Summary data of the peak amplitudes of voltage-gated Na+ currents at 24°C (open bars) and 15°C (closed bars) for type 1a (n = 36), type 1b (n = 15), type 1c (n = 25), type 2a (n = 8), type 2b (n = 15), type 3a (n = 6), and type 3b (n = 6). D) Sample traces of M-currents of a type 1a cell at 24°C and 15°C. E) Sample traces of M-currents of a type 1c cell at 24°C and 15°C. F) Summary data of the peak amplitudes of M-currents at 24°C (open bars) and 15°C (closed bars) for type 1a (n = 27), type 1b (n = 15), type 1c (n = 22), type 2a (n = 8), type 2b (n = 14), type 3a (n = 5), and type 3b (n = 5). G) Summary data of M-currents at 24°C (open bars) for cells displaying multiple firing pattern (n = 56) and phasic firing pattern (n = 39), and M-currents at 15°C (closed bars) for cells displaying multiple firing pattern (n = 58) and phasic firing pattern (n = 37). The neurons of each firing pattern are pooled together from all subtypes in F. Data represent Mean ± SEM, ** P < 0.01, *** P < 0.001; ns, no significant difference.
M-currents were examined in each type of not-for-cold-sensing neurons at 24°C and 15°C (Figure 3D&G). M-currents were present in each cell type at 24°C and were significantly inhibited at 15°C in type 1 and type 2 cells (Figure 3D-F). For example, M-current amplitude in type 1a was 129.6 ± 7.3 pA (n = 27) at 24°C and reduced to 56.3 ± 5.0 pA (n = 27, P < 0.001) at 15°C (Figure 3D&F). Type 1c cells, most of them (80%) were phasic firing, had largest M-currents at the amplitude of 194 ± 17.4 pA (n = 22) at 24°C, and their M-currents were inhibited but the current amplitude remained large at 116.3 ± 9.2 pA at 15°C (n = 22, Figure 1E&F). When M-current amplitudes were compared between cells displaying multiple firing and phasic firing, the latter had significantly larger M-currents (Figure 3G). For example, at 24°C, the M-current amplitude was 118 ± 5.9 pA (n = 56) in multiple firing cells and 172.0 ± 12.3 pA (n = 39, P < 0.001) in phasic firing cells; at 15°C, the M-current amplitude was 59.3 ± 3.4 pA (n = 58) in multiple firing cells and 99.7 ± 7.3 pA (n = 37, P < 0.001) in phasic firing cells.
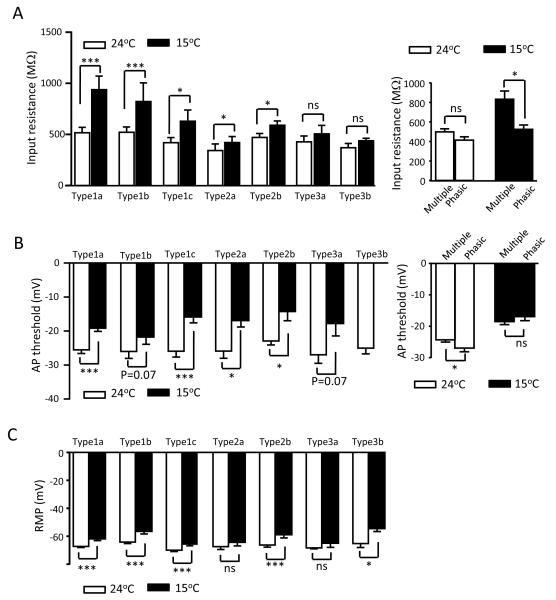
Membrane input resistances, AP thresholds, and resting membrane potentials of each cell type were examined at 24°C and 15°C (Figure 4A-C). Type 1 and type 2 cells but not type 3 cells displayed significant increases in membrane input resistances when these cells were cooled from 24°C to 15°C (Figure 4A left panel). Type 1a cells showed the highest degree of increases in membrane input resistance, from 515.2 ± 53.9 MΩ at 24°C to 938.2 ± 132.7 MΩ at 15°C (n = 36, P < 0.001). When membrane input resistances were compared between the cells displaying multiple firing pattern and phasic firing pattern, no significant difference was found between them at 24°C (Figure 4A right panel). However, membrane input resistances at 15°C were higher in the cells displaying multiple firing pattern (832.7 ± 85.4 MΩ, n = 70) than the cells displaying phasic firing pattern (525.2 ± 45.6 MΩ, n = 39, P <0.05) (Figure 4A right panel). AP thresholds were increased (less negative) in most cell types when cells were cooled down from 24°C to 15°C (Figure 4B). For example, type 1a cells had AP threshold of −25.5 ± 1.1 mV (n = 36) at 24°C and increased to −19.2 ± 0.9 mV (n = 36, P < 0.001) at 15°C. Resting membrane potentials were also increased (less negative) in most cell types except type 2a and type 3a cells (Figure 4B) when cells were cooled down from 24°C to 15°C.
Figure 4. Changes of membrane input resistances, action potential thresholds, and resting membrane potentials in not-for-cold-sensing neurons at the cold temperature of 15°C.
A) Right panel, summary data of membrane input resistances at 24°C (open bars) and 15°C (closed bars) for each cell type. Left panel, summary data of input resistance at 24°C (open bars) for cells displaying multiple AP firing pattern (n = 65) and phasic firing pattern (n = 44), and input resistance at 15°C (closed bars) for cells displaying multiple firing pattern (n = 70) and phasic firing pattern (n = 39). The neurons of each firing pattern are pooled together from all subtypes in left panel. B) Summary data of AP thresholds at 24°C (open bars) and 15°C (closed bars) for each cell type. C) Summary data of resting membrane potentials (RMP) at 24°C (open bars) and 15°C (closed bars) for each cell type. Sample sizes: type 1a cells, n = 36; type 1b cells, n = 15; type 1c cell, n = 25; type 2a cells, n = 8; type 2b cells, n = 15; type 3a cells, n = 5; type 3b cells, n = 5. Data represent Mean ± SEM, * P < 0.05, *** P < 0.001; ns, no significant difference.
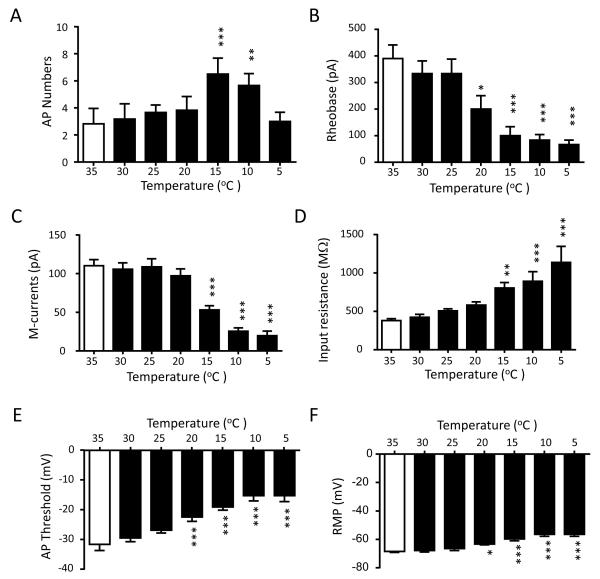
Since type 1a cells showed highest excitability at the cold temperature of 15°C and accounted for the majority of cold-active cells, we further examined their excitability at different temperatures. Figure 5A shows AP firing numbers of type 1a neurons at temperatures of 35, 30, 25, 20, 15, 10 and 5°C. While there was no significant difference in AP firing numbers from 35 to 20°C (2.83 ± 1.14 at 35°C and 3.83 ± 1.01 at 20°C, n = 6), AP firing numbers significantly increased to 6.50 ± 1.18 at 15°C (n = 6, P < 0.001) and to 5.67 ± 0.88 (n = 6, P < 0.05) at 10°C. However, AP firing numbers reduced back to 3.00 ± 0.68 (n = 6) at 5°C (Figure 5A). Rheobase levels were 350 ± 57.7 pA (n = 6) at 35°C and was not significantly different from those measured at the temperatures of 30 and 25°C. However, the rheobase levels were significantly reduced at the temperature of 20°C (n = 6, P <0.05) and bellow (n = 6, P < 0.001) (Figure 5B). For example, the rheobase levels were 200.0 ± 50.0 pA (n = 6) at 20°C and 66.7 ± 16.7 pA (n = 6) at 5°C, significantly lower (P < 0.001) than that of 35°C. We examined M-currents at different temperatures from 35°C to 5°C. While there was no significant difference in M-current amplitudes among recordings performed at 35°C, 30°C, 25°C and 20°C, M-current amplitudes were significantly reduced at the cooling temperatures of 15°C, 10°C, and 5°C (Figure 5C). For example, the amplitude of M-currents was 105.8 ± 8.3 pA (n = 6) at 35°C, and decreased to 53.0 ± 5.4 pA at 15°C (n = 6, P < 0.001), to 25.5 ± 4.4 pA at 10°C (n = 6, P < 0.001), and to 19.8 ± 6.0 pA at 5°C (n = 6, P < 0.001). We examined membrane input resistance at different temperatures from 35°C to 5°C (Figure 5D). Membrane input resistance was 378.5 ± 26.2 MΩ (n = 6) at 35°C and showed temperature-dependent increases with the decreases of temperatures from 35 to 5°C, and statistically significant increases were observed at 15°C and below (Figure 5D). For example, input resistances increased to 804.5 ± 71.2 MΩ (n = 6, P < 0.01) at 15°C and to 1138.0 ± 207.9 MΩ (n = 6, P < 0.001) at 5°C, nearly 2 fold and 3 fold increases, respectively (Figure 5D). Threshold levels of AP firing were examined at different temperatures and were −31.6 ± 2.0 mV (n = 6) at 35°C, and AP threshold levels displayed temperature-dependent increases (less negative) with the decreases of temperatures from 35 to 5°C (Figure 5E). Statistically significant increases in AP threshold levels were observed at 20°C and below (Figure 5E). For example, AP threshold levels were increased to −19.1 ± 1.1 mV (n = 6, P < 0.001) at 15°C and 15.3 ± 2.0 mV (n = 6, P < 0.001) at 5°C. Cooling temperatures had effects on resting membrane potentials in type 1a cells (Figure 5F). At 35°C, resting membrane potentials were −68.5 ± 0.78 mV (n = 6), and were significantly reduced at the temperatures of 20°C and below (Figure 5F). For example, resting membrane potentials decreased to −59.8 ± 1.32 mV at 15°C (n = 6, P < 0.001) and to −56.5 ± 1.42 mV at 5°C (n = 6, P < 0.001).
Figure 5. Electrophysiological properties of cold-active neurons at different temperatures from 35°C to 5°C.
Summary data of electrophysiological parameters at different temperatures from 35°C to 5°C. The parameters are A) AP firing numbers (n = 6), B) rheobase levels (n = 6), C) M-current amplitudes (n = 6), D) membrane input resistances (n = 6), E) AP thresholds (n = 6), and F) resting membrane potentials (n = 6). AP numbers were counted at 2X rheobase levels. M-currents were evoked by the same voltage protocol used in Figure 3D. Data represent Mean ± SEM, * P < 0.05, ** P < 0.01, *** P < 0.001, compared with the values at 35°C.
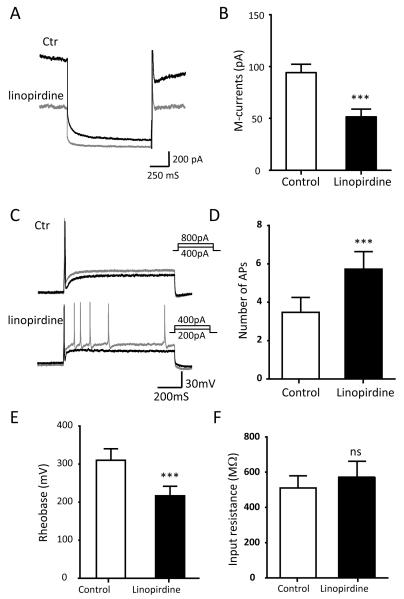
We determined whether pharmacological inhibition of M-currents may change the excitability of not-for-cold-sensing TG neurons. As shown in Figure 6A&B, M-currents were elicited from these TG neurons and the amplitude of M-currents was 94.16 ± 8.20 pA (n = 10) at 24°C. The M-currents were significantly inhibited to 51.58 ± 7.51 pA (n = 10, P < 0.001) by 10 μM linopirdine, a selective M-current inhibitor (Figure 6A&B). The inhibition of M-currents by linopirdine was accompanied by an increase of the excitability of these neurons. This was evidenced by a significant increase in AP firing numbers and a decrease in the rheobase for evoking AP firing (Figure 6C&D). AP firing numbers were increased from 3.47 ± 0.79 in the absence of linopirdine to 5.73 ± 0.90 (n = 15, P < 0.05) in the presence of linopirdine (Figure 6C&D). Rheobase levels were decreased from 310.0 ± 36.6 pA (n = 15) in control in the absence of linopirdine to 216.7 ± 25.2 pA (n = 15) in the presence of linopirdine (Figure 6E&F). However, inhibition of M-currents by linopirdine did not have significant effect on membrane input resistance of these cells (Figure 6F).
Figure 6. Effects on TG neuron excitability by pharmacological inhibition of M-currents.
A) Two sample traces show the M-currents in a cold-active TG neuron in the absence (black line) and presence (gray line) of the M-channel inhibitor linopirdine (10 μM). B) Summary date of the amplitudes of MA-currents in the absence (open bar) and presence (solid bar) of linopirdine (n = 10). C) Sample traces show AP firing in the absence (top) and presence of linopirdine (bottom). Membranes were depolarized by the injection of steps of 50-pA depolarizing currents. Black and gray traces are membrane responses at the rheobase and 2X rheobase levels, respectively. D) Summary data of AP firing numbers in response to the injection of depolarizing currents at 2X rheobase levels in the absence (open bar) and presence of linopirdine (n = 15). E) Summary data of the rheobase for eliciting APs in the absence (open bar) and presence (closed bar, n = 15) of linopirdine. Data represent Mean ± SEM, * P < 0.05, ** P < 0.01, *** P < 0.001.
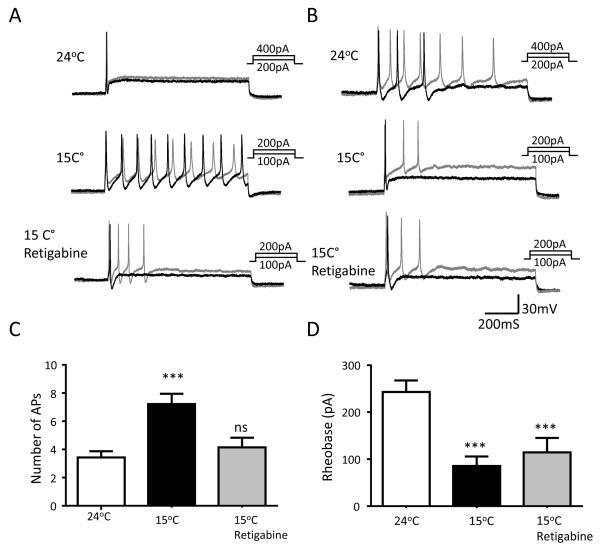
We determined whether cold-induced increases in the excitability of type 1a cold-active TG neurons could be reversed by retigabine, an activator of KCNQ2/3 channels. While the cold temperature of 15°C significantly increased AP firing numbers (3.43 ± 0.44 at 24°C vs 7.21 ± 0.74 at 15°C, n = 14, P < 0.001) in response to membrane depolarization, this effect of the cold temperature was significantly abolished in the presence of 10 μM retigabine (4.14 ± 0.69 at 15°C with retigabine vs 7.21 ± 0.74 at 15°C without retigabine, n = 14, P < 0.001, Figure 7A-C). Interestingly, while the rheobase level was decreased by the cold temperature of 15°C, this effect was not significantly abolished in the presence of retigabine (Figure 7D).
Figure 7. Inhibition of cold-enhanced excitability by M-channel activation in cold-active neurons.
A) A set of sample traces shows that a phasic AP firing cell at 24°C (top panel) becomes multiple AP firing cell at 15°C (middle panel), and the enhanced excitability at 15°C is inhibited in the presence of 10 μM retigabine. AP firing in this cold-active neuron was elicited by the injection of steps of 50-pA depolarizing currents. Black and gray traces in A-B are membrane responses at the rheobase and 2X rheobase levels. B) Similar to A except the cell fired multiple APs at 24°C (top panel) and fired more APs at 15°C (middle panel), and the enhanced excitability is also inhibited in the presence of 10 μM retigabine (bottom panel). C) Summary data of AP firing numbers of cold-active neurons at 24°C (left bar), at the cold temperature of 15°C in the absence (middle bar) and presence (right bar) of 10 μM retigabine (n = 14). AP numbers were counted at 2x rheobase levels. D) Summary data of rheobase levels of cold-active neurons at 24°C (left bar), at the cooling temperature of 15°C in the absence (middle bar) and presence (right bar) of 10 μM retigabine (n = 14). Data represent Mean ± SEM, *** P < 0.001; ns, no significant difference.
Discussion
In the present study we have characterized for the first time at cold temperatures the excitability of small-sized, not-for-cold-sensing TG neurons in the whole-mount TG preparation. We show that at the cold temperature of 15°C most of these not-for-cold-sensing TG neurons become more excitable with increases in the AP firing numbers and/or decreases in the rheobase levels when these neurons are depolarized electrically. We make a new classification for these neurons as cold-active TG neurons (Type 1 cells) since their excitability can be increased by cold temperatures but they are not directly excited to fire APs by cooling temperatures. We further demonstrate in type 1a cells that inhibition of M-currents by cold temperatures contributes to the increases of AP firing numbers, and increases of input resistance by cold temperatures are associated with the reduction of rheobase levels in these neurons at cold temperatures. Our finding that most small-sized, not-for-cold-sensing TG neurons are cold-active neurons is somehow surprising since it is generally thought that neuronal activity is suppressed at cold temperatures.
The increase of TG neuron excitability by cold temperatures shown in the present study is consistent with our previous study conducted using cultured DRG neurons (Sarria et al., 2012). Using the whole-mount TG preparation, the present study for the first time shows that this is a very common feature for small-sized, not-for-cold-sensing TG neurons. The present study also provides new information that is not reported in our previous study. It was unclear in our previous study whether the increases in the excitability of some cultured DRG neurons by cold temperatures was a physiological feature or just a phenomenon of cultured cells because culture conditions could significantly modify intrinsic neuronal excitability. The present study is performed using the whole-mount TG preparation and the TG neurons tested in our study closely represent intact TG neurons in vivo. Therefore, the increase of TG neuron excitability by cold temperatures shown in the present study represents a physiological property of these non-for-cold-sensing TG neurons. Our cold-active TG neurons are most likely to be nociceptive neurons that express high amounts of TTX-resistant VGSNs. This is because VGSN currents in cold-active neurons are only weakly inhibited at cold temperatures, a property of TTX-resistant VGSN currents (Sarria et al., 2012). This property of TTX-resistant VGSNs is important in sensing pain at cold temperatures by nociceptive afferents (Zimmermann et al., 2007). It appears to be also required for displaying the increased excitability at cold temperatures shown in our previous (Sarria et al., 2012) and present studies.
We show that M-currents are present in small-sized, not-for-cold-sensing TG neurons. Interestingly, TG neurons firing multiple APs have significantly smaller M-current amplitude than that of the TG neurons firing single APs. This new finding in TG neurons is consistent with the idea that M-currents play an important role in controlling neuronal excitability and AP firing patterns (Passmore et al., 2003). This role of M-currents is also demonstrated pharmacologically in our study that shows the increases of TG neuron excitability when M-currents are inhibited by linopirdine, a selective M-channel inhibitor. We show that M-currents are significantly inhibited by cold temperatures and that the increase of AP firing numbers in type 1a neurons by cold temperatures is abolished in the presence of the M-current activator retigabine. These new findings suggest that inhibition of M-currents by cold temperatures is a mechanism underlying the increase in the excitability in cold-active TG neurons. However, the inhibition of M-currents by cold temperatures does not seem to account for the decrease of AP rheobase since cold-induced decreases of AP rheobase is not significantly affected in the presence of retigabine. The decrease of AP rheobase by cold temperatures is also unlikely due to the inhibition of IA currents (Sarria et al., 2012).
A possible mechanism underlying the decreases of AP rheobase levels at cold temperatures is the inhibition of leak K+ conductance by cold temperatures. Leak K+ conductance is mainly due to the activity of two-pore domain K+ channels (K2P), including TWIK, TREK, TASK, TALK, THIK, and TRESK channels (Enyedi and Czirjak, 2010). Cold temperatures are known to decrease the activity of K2P channels, resulting in the decreases of leak K+ conductance (Enyedi and Czirjak, 2010). Leak K+ conductance is a major contributing factor of membrane input resistance, and a decrease of leak K+ conductance will lead to an increase in membrane input resistance. Consistently, we show that cold temperatures substantially increase membrane input resistance of the cold-active TG neurons. In contrast, cold temperatures does not have a large effect on the membrane input resistance in the two other cell types. K2P activity is also known to be important for setting resting membrane potentials at more negative levels (Enyedi and Czirjak, 2010). It is predictable that an inhibition of these channels by cold temperatures would reset resting membrane potentials at less negative levels, which is indeed the case in cold-active neurons shown in our study. An enhancement of membrane input resistance would increase the efficiency of a depolarization current to drive membrane voltages toward AP thresholds, and the outcome is the reduction of rheobase level for evoking APs. In addition, resetting membrane potentials at less negative levels by cold temperatures may also contribute in some degree to the decrease of rhoebase levels seen in cold-active neurons.
Our finding that the cold temperatures near 15°C can enhance the excitability of many nociceptive-like not-for-cold-sensing neurons raises a question as whether this effect of cold temperatures may significantly shape sensory modalities, e.g. to increase the responsiveness of these neurons to nociceptive mechanical or chemical stimuli. A future behavioral assessment will be needed to answer this question and to reveal the potential physiological significance of these properties of cold-active neurons at cold temperatures. Interestingly, under pathological conditions such as joint arthritis cold weather can exacerbate joint pain when patients move their joints (Sato, 2003), raising a possibility that the excitability of some not-for-cold-sensing neurons may be enhanced by cold temperatures to account for the enhanced mechanical pain.
Acknowledgements
We thank Dr. Jennifer DeBerry for her comments on an earlier version of this manuscript. This work was supported by NIH grants DE018661 and DE023090 to J.G.G.
References
- Abd-Elsayed AA, Ikeda R, Jia Z, Ling J, Zuo X, Li M, Gu JG. KCNQ channels in nociceptive cold-sensing trigeminal ganglion neurons as therapeutic targets for treating orofacial cold hyperalgesia. Mol Pain. 2015;11:45. doi: 10.1186/s12990-015-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20:2276–2282. doi: 10.1111/j.1460-9568.2004.03695.x. [DOI] [PubMed] [Google Scholar]
- Duan KZ, Xu Q, Zhang XM, Zhao ZQ, Mei YA, Zhang YQ. Targeting A-type K(+) channels in primary sensory neurons for bone cancer pain in a rat model. Pain. 2012;153:562–574. doi: 10.1016/j.pain.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- Johnston J, Forsythe ID, Kopp-Scheinpflug C. Going native: voltage-gated potassium channels controlling neuronal excitability. J Physiol. 2010;588:3187–3200. doi: 10.1113/jphysiol.2010.191973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R, de la Pena E, Donovan-Rodriguez T, Belmonte C, Viana F. Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Neurosci. 2009;29:3120–3131. doi: 10.1523/JNEUROSCI.4778-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain. 2005;1:16. doi: 10.1186/1744-8069-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Morin C, Bushnell MC. Temporal and qualitative properties of cold pain and heat pain: a psychophysical study. Pain. 1998;74:67–73. doi: 10.1016/S0304-3959(97)00152-8. [DOI] [PubMed] [Google Scholar]
- Passmore GM, Selyanko AA, Mistry M, Al-Qatari M, Marsh SJ, Matthews EA, Dickenson AH, Brown TA, Burbidge SA, Main M, Brown DA. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci. 2003;23:7227–7236. doi: 10.1523/JNEUROSCI.23-18-07227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Phuket TR, Covarrubias M. Kv4 Channels Underlie the Subthreshold-Operating A-type K-current in Nociceptive Dorsal Root Ganglion Neurons. Front Mol Neurosci. 2009;2:3. doi: 10.3389/neuro.02.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:13373–13378. doi: 10.1073/pnas.231376298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Babes A, Pluteanu F. A cold- and menthol-activated current in rat dorsal root ganglion neurones: properties and role in cold transduction. J Physiol. 2002;545:595–614. doi: 10.1113/jphysiol.2002.024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Flonta M. Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurones. Neurosci Lett. 2001;297:171–174. doi: 10.1016/s0304-3940(00)01694-3. [DOI] [PubMed] [Google Scholar]
- Rose K, Ooi L, Dalle C, Robertson B, Wood IC, Gamper N. Transcriptional repression of the M channel subunit Kv7.2 in chronic nerve injury. Pain. 2011;152:742–754. doi: 10.1016/j.pain.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarria I, Ling J, Gu JG. Thermal sensitivity of voltage-gated Na+ channels and A-type K+ channels contributes to somatosensory neuron excitability at cooling temperatures. J Neurochem. 2012;122:1145–1154. doi: 10.1111/j.1471-4159.2012.07839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato J. Weather change and pain: a behavioral animal study of the influences of simulated meteorological changes on chronic pain. Int J Biometeorol. 2003;47:55–61. doi: 10.1007/s00484-002-0156-9. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27:13680–13690. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing H, Ling J, Chen M, Gu JG. Chemical and cold sensitivity of two distinct populations of TRPM8-expressing somatosensory neurons. J Neurophysiol. 2006;95:1221–1230. doi: 10.1152/jn.01035.2005. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hatakeyama T, Taniguchi K. Immunohistochemical colocalization of TREK-1, TREK-2 and TRAAK with TRP channels in the trigeminal ganglion cells. Neurosci Lett. 2009;454:129–133. doi: 10.1016/j.neulet.2009.02.069. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Fang D, Liu M, Cai J, Wan Y, Han JS, Xing GG. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain. 2013;154:434–448. doi: 10.1016/j.pain.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447:855–858. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]