Abstract
The VacA toxin secreted by Helicobacter pylori enhances the ability of the bacteria to colonize the stomach and contributes to the pathogenesis of gastric adenocarcinoma and peptic ulcer disease. The amino acid sequence and structure of VacA are unrelated to corresponding features of other known bacterial toxins. VacA is classified as a pore-forming toxin, and many of its effects on host cells are attributed to formation of channels in intracellular sites. The most extensively studied VacA activity is its capacity to stimulate vacuole formation, but the toxin has many additional effects on host cells. Multiple cell types are susceptible to VacA, including gastric epithelial cells, parietal cells, T cells, and other types of immune cells. This review focuses on the wide range of VacA actions that are detectable in vitro, as well as actions of VacA in vivo that are relevant for H. pylori colonization of the stomach and development of gastric disease.
Keywords: bacterial toxins, vacuolating toxin, autotransporter, type V secretion, gastric cancer
1. Introduction
Helicobacter pylori was first cultured from human gastric tissue in 1983 [1]. Several years later, it was reported that H. pylori broth culture supernatants contained a proteinaceous component known as “vacuolating cytotoxin”, which, when added to cultured eukaryotic cells, caused the cells to become vacuolated [2]. Bacterial toxins with similar activity had not been described previously, and initially there was controversy about whether or not a H. pylori vacuolating toxin actually existed. Subsequent studies revealed the identity of the vacuolating toxin (termed VacA) [3,4,5,6,7] and showed that it has properties and activities substantially different from those of other bacterial toxins.
2. Features of vacA and Related Genes in Helicobacter Species
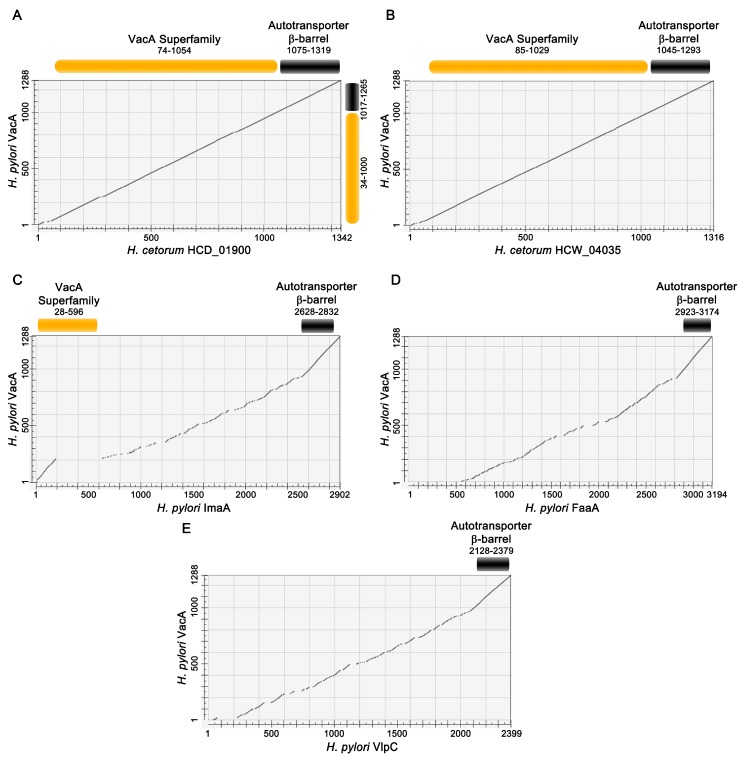
All H. pylori strains contain a single chromosomal vacA gene. The intact H. pylori vacA gene encodes a protein about 140 kDa in mass. The genus Helicobacter includes at least 20 different species, but intact vacA genes are present only in H. pylori and H. cetorum, a species isolated from stomachs or fecal contents of marine mammals [8]. Similar to the association between H. pylori and gastritis in humans, H. cetorum is associated with gastritis in cetaceans and perhaps pinnipeds (seals) [9,10]. Genome sequence analysis of H. cetorum strains from a dolphin and a whale revealed the presence of an intact vacA gene next to cysS [8], consistent with the linkage of vacA and cysS in H. pylori. The proteins encoded by these H. cetorum vacA genes exhibit about 60%–70% protein-level identity to the most closely related H. pylori vacA gene product, and about 66% identity to one another (Figure 1A,B). The dolphin isolate of H. cetorum also contains an extra triplet of divergent vacA genes [8]. It is not yet known whether H. cetorum VacA proteins elicit cytotoxic effects similar to those described for H. pylori VacA.
Figure 1.
Relatedness of H. pylori VacA to H. cetorum VacA proteins and H. pylori VacA-like proteins. The amino acid sequence of VacA (WP_000405515) from a representative H. pylori strain (J99) was aligned to the sequences of related proteins using Needleman–Wunsch global alignment. The amino acid numbers of each protein are shown on the x and y axes, and results of the pairwise alignments are represented as dot matrices. Protein sequences also were searched against the Conserved Domain Database, and domains shared between VacA and the query protein sequence are shown above the dot matrices. Query proteins were H. cetorum HCD_01900 (WP_014658932) (A); H. cetorum HCW_04035 (WP_014660950) (B); H. pylori ImaA (WP_000808594) (C); H. pylori FaaA (WP_000222280) (D); and H. pylori VlpC (WP_000874591) (E). The sequences of the latter three H. pylori VacA-like proteins are from H. pylori strain J99. “VacA superfamily” corresponds to the VacA passenger domain, and “autotransporter β-barrel” corresponds to a domain predicted to be localized to the outer membrane. The autotransporter β-barrel is conserved in all of the proteins analyzed, but there is very little sequence relatedness when comparing the passenger domain of H. pylori VacA with corresponding regions of H. pylori VacA-like proteins.
Fragmented vacA pseudogenes are found in H. acinonychis, a Helicobacter species isolated from cheetahs and other large cats [11,12]. Whole genome sequencing of one strain revealed the presence of two nearly identical vacA pseudogenes [12]. The gene duplication presumably occurred after the vacA gene was disrupted. A protein encoded by a precursor of the H. acinonychis vacA pseudogene has been reconstructed in silico and exhibits about 64% amino acid identity to its closest match in H. pylori [13].
H. pylori and several other Helicobacter species (including H. bilis, H. heilmannii, H. ailurogastricus, H. felis, H. bizzozeronii, H. suis, H. acinonychis, and H. cetorum) contain “vacA-like” genes. This designation is a bit of a misnomer as the similarity between VacA and these “VacA-like” gene products is limited mainly to the C-terminal ends of the proteins (Figure 1C–E). This C-terminal region is required for secretion of H. pylori VacA [5,6,14], but is not part of the soluble VacA toxin. To our knowledge, the three vacA-like genes from H. pylori are the only vacA-like genes that have been studied experimentally. In H. pylori, these genes are designated imaA (immunomodulatory autotransporter A, HP0289), faaA (flagella-associated autotransporter A, HP0609/0610), and vlpC (VacA-like protein C, HP0922) [15,16]. Each of the vacA-like genes enhances the capacity of H. pylori to colonize the stomach in rodent models [15,16,17,18], and transcription of each gene is upregulated in the gastric environment compared to the level of transcription during bacterial growth in vitro [15,16,19]. The H. pylori VacA-like proteins localize to the bacterial surface, where ImaA and VlpC localize to a bacterial pole and FaaA localizes to the flagella [15,16,20]. Little is known about the functions of these three proteins, but analysis of mutant strains has provided clues. Specifically, analyses of a faaA mutant revealed mislocalization of the flagella and decreased bacterial motility [16]; gastric epithelial cells co-cultured with an imaA mutant produce higher levels of IL-8 and TNF-α than cells co-cultured with wild-type H. pylori [15]; mutations in vlpC have been associated with high-level resistance to metronidazole [21].
3. VacA Transcription, Regulation, and Secretion
The H. pylori vacA transcriptional start site is located about 120 nucleotides upstream from the ATG start codon [5,22]. A stem-loop structure in the 5′ untranslated region (UTR) of the vacA transcript stabilizes the vacA mRNA, particularly during conditions of environmental stress [23]. The transcription of vacA is regulated in response to growth phase, with the highest levels of transcription occurring in late log phase [24,25]. There has been relatively little in-depth analysis of vacA regulation in response to environmental conditions, but some studies suggest that vacA transcription is regulated in response to low pH, iron concentration, salt concentration, and bacterial contact with host cells [23,26,27,28].
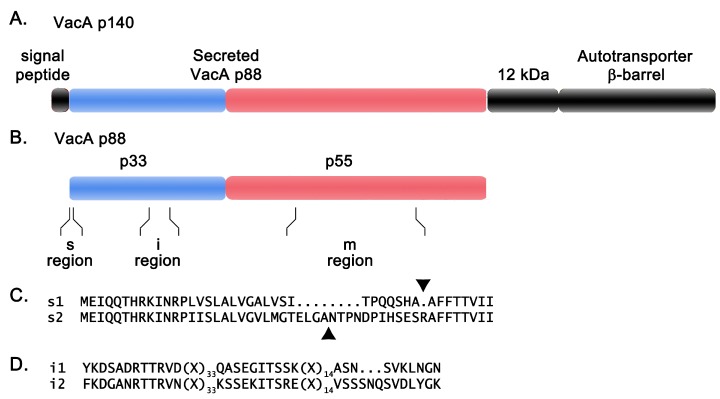
VacA is translated into a 140 kDa protein, which undergoes Sec-dependent cleavage of an amino-terminal signal sequence and carboxy-terminal proteolytic cleavage [4,5,6] (Figure 2A). Cleavage yields an active toxin about 88 kDa in mass, as well as a ~12 kDa peptide and a ~33 kDa protein with a predicted β-barrel structure (autotransporter β-barrel) [3,4,5,6,29,30]. The protease responsible for carboxy-terminal proteolytic cleavage has not been identified. The 88 kDa toxin molecules are translocated across the outer membrane and then can be either released into the extracellular space as soluble proteins (along with the 12 kDa peptide) [3,25,29,30] or retained on the bacterial surface [20,31]. The 33 kDa autotransporter β-barrel localizes to the outer membrane, and is required for secretion of the 88 kDa toxin [6,14]. These features suggest that VacA is secreted by an autotransporter or type V mode of secretion [4,5,14].
Figure 2.
VacA organization and genetic diversity. (A) The organization of the 140 kDa VacA protein is shown, including the amino-terminal signal peptide, the secreted 88 kDa VacA toxin, a 12 kDa peptide of unknown function, and a carboxy-terminal domain with a predicted β-barrel structure (autotransporter β-barrel); (B) The 88 kDa secreted VacA toxin can undergo proteolytic cleavage into two domains, p33 and p55 (colored blue and red). Three regions of sequence diversity (s-, i-, and m-regions) are shown; (C) Representative signal peptides from type s1 and s2 VacA proteins are shown. Arrowheads mark the sites of signal peptide cleavage. Secreted type s2 VacA proteins contain an amino-terminal extension relative to the secreted type s1 VacA proteins; (D) Representative i-region sequences are shown.
4. Membrane Channel Formation by VacA
Many bacterial toxins enter host cells and cause alterations by exerting an enzymatic activity in intracellular sites, and others act by forming pores in the plasma membrane of host cells. VacA enters host cells, but is not known to have any enzymatic activity. Based on its capacity to form anion-selective channels in planar lipid bilayers [32,33,34], VacA has been classified as a pore-forming toxin. VacA channels can conduct chloride, bicarbonate, and small organic molecules [32,33,34,35,36], and have properties similar to those of ClC channels in host cells [37]. VacA mutant proteins lacking the ability to form a membrane channel in artificial bilayers lack vacuolating toxic activity in cell culture assays [38,39]. Similarly, chemical inhibitors of chloride channels impair VacA channel activity in lipid bilayers and impair vacuolation in cell culture [33,35,36]. Notably, the chemical inhibitors used for these experiments might not act exclusively on VacA channels, but might also act on other cellular targets. Most evidence indicates that the effects of VacA on host cells are directly attributable to channel formation by VacA, rather than activation of endogenous cellular channels.
5. VacA Structure
The 88 kDa secreted VacA protein can undergo limited proteolysis in the presence of trypsin or during prolonged storage to yield 33 kDa and 55 kDa fragments (Figure 2B) [6,40]. These are considered to be two domains of VacA (p33 and p55). Mixtures of recombinant p33 and p55 proteins can reconstitute a functionally active form of VacA [41,42]. The p55 domain has a predominantly β-helical structure [43], which is a feature shared by the passenger domains of several proteins secreted by an autotransporter mechanism in other Gram-negative bacterial species [44]. No high resolution structural data are available for the p33 domain. The amino acid sequence of the 88 kDa secreted VacA protein is not closely related to sequences of any other known bacterial toxins.
Both p33 and p55 are required for efficient binding of the toxin to the plasma membrane when VacA is added externally to cells [41]. The p33 domain is required for insertion of VacA into membranes to form anion-selective channels [38,39]. When expressed intracellularly, the minimal VacA region required for cell vacuolation encompasses residues 1–422, which includes the entire p33 domain plus 111 amino acids from the amino-terminal portion of the p55 domain [45,46,47].
The N-terminus of the p33 domain contains a sequence of 32 uncharged amino acids, corresponding to the only predicted hydrophobic segment within VacA long enough to span a membrane [38,39,48]. Deletion of this region results in a VacA mutant lacking cell-vacuolating activity and defective in membrane channel formation in planar lipid bilayers [38]. The amino-terminal hydrophobic region of VacA includes three tandem GXXXG transmembrane association motifs (defined by glycine residues at positions 14, 18, 22, and 26) [39,48,49]. Mutagenesis of amino acids within this region, including glycine residues at positions 14 and 18 or a proline residue at position 9, abolishes VacA channel formation and vacuolating activity [39,50,51].
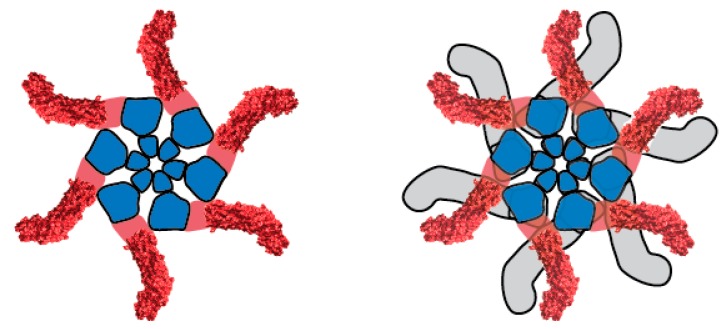
VacA oligomerizes in solution to form an assortment of flower- or snowflake-shaped structures [52,53,54]. These include double-layered structures (dodecamers and tetradecamers) as well as single layered structures (mainly hexamers and heptamers, but occasionally higher order forms) (Figure 3). Several lines of evidence indicate that oligomerization is required for VacA activity [38,55,56,57,58]. The structure of water-soluble, single layered VacA oligomers is proposed to resemble the structure of VacA membrane channels.
Figure 3.
Structural organization of water-soluble VacA oligomers. A hexamer (left) and dodecamer (right) are shown. Within each component p88 monomer, p33 and p55 domains are shown in blue and red, respectively. A crystal structure has been solved for a portion of p55 [43], corresponding to peripheral elements of the oligomer [54]. Water-soluble hexamers are predicted to be structurally similar to membrane channels formed by VacA.
Exposure of VacA oligomers to acidic pH or alkaline pH results in the disassembly of VacA oligomers into monomers [59,60]. When added to cultured cells, preparations of VacA exposed to low pH or high pH have greatly increased cytotoxic activity compared to preparations of oligomeric VacA [61,62]. Therefore, it is thought that VacA first interacts with the plasma membrane of host cells as a monomer, where it oligomerizes and inserts to form a functional membrane channel.
6. VacA Diversity among H. pylori Strains
Early studies noted that there is considerable variation in vacuolating toxin activity among H. pylori strains [2]. One contributing factor is variation among strains in VacA transcription [63]. There is also variation among strains in levels of VacA protein secretion [3]. At present, it is not known whether differences among strains in levels of VacA secretion are primarily due to differences in VacA transcription, differences in transcript stability, or differences in the efficiency with which various forms of VacA are secreted.
All H. pylori strains contain a vacA gene, and in most strains the vacA open reading frame is intact. Frameshift mutations in vacA are present in a small proportion of isolates [64], resulting in an absence of VacA protein production. Phylogenetic analysis of vacA sequences from a large number of H. pylori strains has revealed the existence of several distinct groups of vacA alleles, several of which have distinct geographical distributions [13]. For example, vacA alleles found in many H. pylori strains isolated in East Asia are considerably different from the vacA alleles typically found in strains isolated in Europe or Africa [13,65]. Divergence among groups of vacA alleles is principally due to positively selected sequence changes in the region of vacA encoding the p55 domain, and corresponds to surface-exposed sites in the p55 crystal structure [13].
Three main regions of diversity in VacA sequences have been recognized: the signal sequence region (s-region), the intermediate region (i-region), and middle region (m-region) [66,67] (Figure 2B). Within each of these regions, sequences can be classified into two main types (s1 or s2, i1 or i2, and m1 or m2). The s-region of VacA corresponds to the amino-terminal signal sequence and the amino-terminus of the secreted toxin (Figure 2C) [66,68,69,70]. The i-region is found within the p33 domain of VacA (Figure 2D) [67]. The m-region corresponds to part of the p55 domain [66]. Homologous recombination occurs commonly in H. pylori, and consequently, vacA alleles can contain multiple possible combinations of s-, i- and m-region types (s1-i1-m1; s2-i2-m2; s1-i1-m2, etc.) [66].
In contrast to type s1 forms of VacA, type s2 forms of VacA do not cause vacuolation of mammalian cells [66,68,69,70]. This dichotomy in activity is attributable to different sites of signal sequence cleavage in s1 and s2 VacA proteins. Specifically, a hydrophilic 12 amino acid N-terminal extension is present in type s2 proteins, but absent from type s1 proteins [66,68,69,70]. Several studies have reported that type m1 and m2 forms of VacA exhibit distinct cell-type specificities. For example, the activity of type m1 VacA toward HeLa cells is greater than the activity of type m2 VacA toward these cells, whereas both m1 and m2 forms are highly active toward RK-13 cells [71,72]. The difference in activity of m1 and m2 VacA proteins has been attributed to cell-type dependent variations in VacA binding to cells [71], and may also be attributable to differences in channel-forming properties [73]. Cell type specificity has been mapped to a 148-residue segment within the m-region of VacA [72]. One study reported that the i-region (within the p33 domain) is a determinant of vacuolating toxin activity in strains that produce type s1-m2 forms of VacA [67]. Type i1 forms of VacA are more active than type i2 forms of VacA in experiments conducted with Jurkat T cells [74].
Epidemiological studies have demonstrated a correlation between the type of vacA allele present in H. pylori strains and the risk of gastroduodenal disease. Specifically, the risk of gastric cancer or peptic ulcer disease is higher in persons infected with strains containing type s1, i1 or m1 forms of vacA, compared to persons infected with strains containing type s2, i2 or m2 forms of vacA [66,67,75,76]. The increased risk of disease observed with strains containing s1, i1, or m1 forms of vacA is probably attributable not only to the effects of VacA, but also to the effects of additional virulence determinants. Specifically, strains containing a type s1 vacA allele typically harbor the cag pathogenicity island (which encodes several important virulence determinants, including CagA and a type IV secretion system) and the outer membrane protein adhesin BabA, whereas strains containing a type s2 vacA allele typically lack the cag pathogenicity island and often lack babA [76].
7. VacA Activities in Cell Culture Systems
7.1. Effects on Epithelial Cells
7.1.1. Endosomal Alterations
The most extensively studied activity of VacA is its ability to cause vacuolation of cultured cells. Most studies of this phenomenon and other VacA activities have been conducted with the highly active s1-i1-m1 form of VacA. Vacuolation can be observed within a few hours after addition of VacA to cells and is enhanced by the presence of weak bases [77]. The membranes of VacA-induced vacuoles contain markers typically found in membranes of late endosomes (LEs) [78,79,80,81], which suggests that the vacuoles arise from late endosomal compartments [82]. The current model for VacA-induced vacuolation [83,84] proposes that the secreted monomeric form of VacA binds to the plasma membrane. Upon binding, VacA monomers form oligomers, which are trafficked to LEs, where they form anion-selective channels in the LE membrane [32,33,34,35,36]. Transit of chloride ions through VacA channels in the LE membrane leads to an increase in intraluminal chloride concentration, which in turn triggers the enhancement of V-ATPase proton pumping activity and a decrease in intraluminal pH. Membrane-permeant weak bases diffuse into LEs, where they are protonated in the acidic environment and trapped. As a result, LEs undergo osmotic swelling, resulting in cell vacuolation [85,86,87]. In addition to causing cell vacuolation, VacA causes a variety of functional alterations related to disruption of proper endocytic compartment trafficking. These include inhibition of intracellular degradation of epidermal growth factor [88], inhibition of procathepsin D maturation [88], perturbation of transferrin recycling [89], and in immune cells, inhibition of antigen presentation [90].
7.1.2. Autophagy
When added to cultured gastric epithelial cells, VacA induces autophagy [91], the regulated degradation and recycling of cellular components in the cytoplasm. VacA is necessary and sufficient for H. pylori-induced autophagy [91]. Similar to VacA-induced vacuole formation, VacA-induced autophagy is dependent on the capacity of VacA to form membrane channels [91], but the autophagosomes formed in response to VacA are distinct from the more abundant and larger intracellular vacuoles that form in response to VacA [91]. Although the mechanisms by which VacA induces autophagy are not fully understood, VacA-induced autophagy has been shown to depend on binding of VacA to low-density lipoprotein receptor-related protein 1 (LRP1) [92]. Inhibition of autophagy leads to increased stability of intracellular VacA and increased cell vacuolation [91]. One hypothesis is that the induction of autophagy upon exposure to VacA is a response initiated by the host cell to degrade VacA and prevent additional toxin-induced cell damage [91]. Although acute exposure of host cells to VacA induces autophagy, prolonged exposure of host cells to VacA has been shown to disrupt autophagy [93,94].
7.1.3. Mitochondrial Alterations
Treatment of cells with VacA results in an assortment of mitochondrial alterations, including reduction of mitochondrial transmembrane potential [95,96,97], release of cytochrome c [97,98,99], activation of Bax and Bak [97,100], and mitochondrial fragmentation [100]. After entry into host cells, VacA can localize to mitochondria [96,98,100,101], leading to the hypothesis that the toxin acts directly on mitochondria. In support of this model, VacA can cause a reduction in the transmembrane potential of isolated mitochondria [97], and VacA is imported into the inner mitochondrial membrane (IMM) [98,102,103]. The ability of VacA to induce mitochondrial dysfunction is dependent on VacA channel activity [96,99,100]. Thus, one model proposes that VacA is imported into mitochondria and induces mitochondrial transmembrane potential reduction, perhaps by pore formation. This depolarization stimulates an initial release of cytochrome c, the activation of Bax/Bak, and the subsequent Bax/Bak-dependent release of cytochrome c. Another hypothesis is that VacA-induced mitochondrial dysfunction is due to indirect actions of VacA. For example, VacA may act indirectly by activating pro-apoptotic factors to trigger mitochondrial-dependent apoptosis [97].
7.1.4. Epithelial Barrier Alterations
When added to cultured epithelial cells, VacA causes increased plasma membrane permeability, resulting in efflux of various anions and other small molecules, including chloride, urea, and bicarbonate, into the extracellular space [104,105]. VacA-induced permeabilization of cells is attributed to the formation of VacA channels in the plasma membrane [33,34,36,105]. In addition to causing increased permeability of the plasma membrane, VacA causes increased paracellular permeability of polarized monolayers [106,107,108]. The mechanism by which VacA causes increased paracellular permeability is not well understood.
7.1.5. Altered Cell Signaling
Several cellular alterations can be detected very rapidly after exposure of cells to VacA, and are likely due to the binding of VacA to the surface of host cells. In both gastric epithelial cells [109,110] and T cells [111], VacA activates p38, a mitogen-activated protein (MAP) kinase. VacA-induced activation of the p38 signaling pathway leads to induction of cyclooxygenase 2 (COX-2) expression, which results in enhanced prostaglandin E2 production [110]. VacA-induced activation of the p38 signaling pathway also can lead to the activation of activating transcription factor 2 (ATF-2) [109]. VacA can also activate another MAP kinase, ERK1/2 [109]. In addition to activating MAP kinases, VacA can activate a signaling pathway that activates G protein-coupled receptor kinase interactor (Git1) [112], a signaling pathway that leads to the upregulation of vascular endothelial growth factor (VEGF) [113], and the β-catenin signaling pathway [114]. The VacA cell surface receptors required for activating most of these pathways have not been characterized, but RPTP-β is reported to be the VacA receptor required for activation of Git1 [112] and epidermal growth factor receptor is reported to be required for upregulation of VEGF [113].
7.1.6. Cell Death
VacA-induced cell vacuolation is not a cytolethal alteration [2], but exposure of epithelial cells to VacA can potentially result in cell death [101,115,116]. AZ-521 cells (which are of duodenal origin) are particularly susceptible to VacA-induced cell death [117,118]. VacA-induced cell death is preceded by an assortment of mitochondrial alterations [95,96,97,98,99,100,119], which suggests that these alterations are mechanistically important in the process by which VacA causes cell death. VacA reduces the expression of pro-survival factors [120] and causes endoplasmic reticulum (ER) stress [121], which could also contribute to VacA-induced cell death. VacA can cause cell death by both apoptosis and necrosis [117].
7.2. Effects on Immune Cells and Parietal Cells
7.2.1. Effects on Immune Cells
VacA can alter the function of many types of immune cells [84,122], including lymphocytes, macrophages, eosinophils [123,124], mast cells [125,126], and dendritic cells [127,128]. VacA inhibits activation and proliferation of T cells and B cells [111,129,130,131], and can interfere with antigen presentation in B cells [90]. In macrophages, VacA contributes to the formation of large vesicles termed megasomes, and impairs the maturation and function of vesicular compartments [132,133]. VacA alters various signal transduction pathways in macrophages [134,135], and can cause macrophage apoptosis [136]. These VacA-induced effects may impair the ability of macrophages to engulf H. pylori. In addition to immunosuppressive effects, VacA stimulates the expression of the proinflammatory enzyme COX-2 in macrophages and neutrophils [111].
7.2.2. Effects on Parietal Cells
Two studies reported that VacA inhibits gastric acid secretion from parietal cells [137,138]. In one study, exposure of parietal cells to VacA resulted in permeabilization of the plasma membrane and calcium influx that ultimately caused the disruption of actin arrangement in apical microvilli and an inhibition of acid secretion [138]. At present, it is not known whether this effect of VacA on parietal cells contributes to a reduction in gastric acid secretion that is sometimes observed in the course of H. pylori infection.
8. Binding, Internalization, and Intracellular Trafficking of VacA
8.1. Cell Surface Binding and Receptors
Various studies have reached differing conclusions about whether VacA binding to cells is saturable [139,140,141] or nonsaturable [62,142,143]. Therefore, it is unclear whether VacA binds to a single abundant, low-affinity receptor or to multiple cell surface components. Multiple putative VacA receptors on the surface of epithelial cells have been reported, including both protein and lipid receptors. These include receptor protein tyrosine phosphatases (RPTP) α and β [60,112,144,145], low-density lipoprotein receptor-related protein-1 (LRP1) [92], epidermal growth factor receptor (EGFR) [146], heparan sulphate [147], sphingomyelin [148,149], glycosphingolipids [150], and phospholipids [151]. Among these putative receptors, sphingomyelin is the only plasma membrane component whose presence or absence is a functionally important determinant of sensitivity of epithelial cells to VacA, and also an important determinant of the extent to which VacA binds to the cell surface [148]. Binding of VacA to sphingomyelin probably accounts for localization of the toxin to lipid rafts [143,152,153]. Although several putative receptors for VacA have been identified on epithelial cells, β2 integrin subunit (CD18) is the only VacA receptor that has been identified on T cells [154].
VacA binding to RPTP-β triggers alterations in cell signaling that ultimately lead to gastric tissue damage [112]. Correspondingly, oral administration of VacA to wild-type mice results in gastric damage, whereas RPTP-β knockout mice are resistant to VacA-induced gastric damage [112]. RPTP-β is not the sole receptor for VacA, as VacA is still internalized into epithelial cells in RPTP-β knockout mice [112]. VacA binding to LRP1 is important for VacA-induced autophagy and apoptosis [92].
8.2. Pore Formation at the Cell Surface
After binding to the cell surface, VacA increases plasma membrane permeability and causes membrane depolarization [36,39]. These alterations are attributed to insertion of VacA into the plasma membrane and formation of anion-selective membrane channels [32,33,34,35,36]. It is proposed that the toxin can also form channels in intracellular sites (endosomes and mitochondria). Relatively little is known about the relationships between VacA channel formation and intracellular trafficking of the toxin.
8.3. VacA Internalization and Intracellular Trafficking
Upon binding to the cell surface, VacA is internalized by a clathrin-independent, Cdc42 dependent, and Rac1 dependent route that requires actin polymerization [155,156,157,158]. Within 10 min after internalization, VacA is found in glycosylphosphatidyl inositol anchored protein (GPI-AP)-enriched early endosomal compartments (GEECs), within 30 min in early endosomes (EEs), and within 2 h in LEs [156,157].
Several studies have provided evidence that the intracellular localization of VacA is not limited to endosomal compartments. As one example, VacA has been detected in association with mitochondria in host cells [96,119]. The mechanisms by which VacA traffics to mitochondria are not well understood. One model proposes that a subset of VacA-containing endosomes co-localize with mitochondria, and that VacA is transferred directly from endosomes to mitochondria [119]. In support of this model, VacA causes cellular changes that result in the co-fractionation of endosomes with mitochondria [119]. Another model proposes that VacA is released into the cytosol and is imported into mitochondria via mitochondrial import proteins [102,103]. Although VacA gains access to the cytosol if expressed in host cells or microinjected into cells [45,98], it is not known whether VacA added externally to cells can ultimately gain access to the cytosol (either by directly crossing the plasma membrane or by release from endosomes). It has been suggested that VacA may travel retrograde through the Golgi and ER [81], but this has not been investigated in detail. Further studies are needed to better understand VacA trafficking within host cells.
9. Activity of VacA in Animal Models
Animal models have been utilized to investigate a potential role of VacA in promoting H. pylori colonization of the mammalian stomach. VacA mutant strains of H. pylori can colonize mice, gerbils, and gnotobiotic piglets [159,160,161,162], which indicates that VacA is not essential for gastric colonization. However, when coinfections are performed with isogenic wild-type and VacA mutant strains, VacA mutant strains are at a competitive disadvantage [162,163]. In addition, ΔvacA mutant strains colonize mice less efficiently or at reduced levels compared to wild-type strains [128,162,163].
Experiments in animal models have also revealed a role for VacA in promoting gastric pathology. Oral or intragastric administration of purified VacA to mice results in damage to the gastric mucosa and recruitment of inflammatory cells [6,112,125,164]. Based on the capacity of VacA to induce ulceration in mice when administered intragastrically, it has been suggested that VacA contributes to the pathogenesis of gastric ulceration in humans. The intragastric concentration of VacA in animals receiving intragastrically administered VacA substantially exceeds the 20–800 pg per mL concentration of VacA estimated to be present in gastric juice, based on a bead-based ELISA performed on samples from H. pylori-positive patients [165], but local concentrations of VacA at sites of H. pylori interaction with gastric epithelial cells might be considerably higher than concentrations in gastric juice. In studies of gerbils experimentally infected with H. pylori, vacA mutant strains are less likely than isogenic wild-type strains to produce gastric ulcers [161]. In addition, colonization studies in mice indicate that H. pylori strains producing forms of VacA that are most active in vitro (s1-i1) induce more severe and extensive metaplasia and inflammation in the stomach than strains producing forms of VacA that are less active in vitro (s1-i2 or s2-i2) [163]. Another study showed that, in comparison to a wild-type strain, ΔvacA mutants induce stronger T-helper 1 (Th1) and T-helper 17 (Th17) responses and trigger more severe gastric pathology in mice [128]. This latter observation may be attributable to immunomodulatory activities of VacA.
H. pylori-infected humans often develop serum and gastric mucosal antibody responses to VacA (as well as many other H. pylori antigens) [166,167], but these humoral immune responses do not result in clearance of H. pylori infection. In contrast, immunization of animals with VacA provides protective immunity against subsequent challenge with H. pylori [168,169,170,171,172,173]. VacA has also been used as a component of vaccines used for therapeutic immunization (i.e., immunization designed to promote clearance of H. pylori infection) [169,171,173].
The presence of H. pylori in humans is inversely correlated with the incidence of allergy and asthma [174,175]. Studies in mice have shown that H. pylori infection protects against development of allergic asthma, and that the protective effect of H. pylori is attributed to tolerogenic reprogramming of dendritic cells [176]. VacA (and also gamma-glutamyl transpeptidase, GGT) contribute to the ability of H. pylori to induce tolerizing effects on murine dendritic cells in vitro and in vivo [128,177] and VacA is required for protection against allergic asthma in a mouse model [128,177].
10. Comparisons of VacA Activities in Vitro and in Vivo
VacA causes a wide spectrum of alterations in multiple cell types in vitro. One might presume that some VacA activities observed in vitro are more relevant in vivo than others. Therefore, there is considerable interest in defining VacA activities that are most relevant in vivo for promoting H. pylori colonization of the stomach or the development of diseases such as peptic ulceration or gastric cancer.
Cell vacuolation is one of the most extensively studied VacA-induced phenomena in vitro. Human gastric epithelial cells are susceptible to VacA [178,179] and vacuolation is occasionally observed in epithelial cells from gastric biopsies [180], albeit less prominently than in cultured cells treated with VacA. One study reported that VacA can promote intracellular survival of H. pylori in gastric epithelial cells [181]. Conversely, H. pylori is predominantly an extracellular organism and does not replicate within intracellular vacuoles [182]. Therefore, it is difficult to envision a mechanism by which vacuole formation per se would be relevant for H. pylori colonization of the stomach or H. pylori-associated gastric diseases. On the other hand, alterations in host cells resulting from VacA-induced perturbation of endocytic trafficking could be advantageous for the bacteria (for example, by inhibiting antigen presentation) [90].
One plausible action for VacA in vivo is to enhance the availability of nutrients or essential growth factors (such as metals), thereby promoting growth of H. pylori. Nutrients could be released through VacA channels in the plasma membrane or by VacA-induced changes in paracellular permeability. One study provided evidence that VacA perturbs transferrin recycling in host cells, leading to enhanced availability of iron and enhanced growth of H. pylori on the surface of epithelial cells [89]. VacA-induced cell death could also result in release of nutrients.
VacA interferes with the functions of several types of immune cells in vitro, and this could also be an important action of the toxin in vivo. Specifically, by interfering with the normal functions of T cells, B cells, neutrophils, macrophages, and eosinophils, VacA may attenuate the host immune response and thereby facilitate persistent H. pylori colonization of the stomach.
H. pylori strains producing s1 forms of VacA, which are most active in vivo, typically contain the cag pathogenicity island, whereas strains producing s2 forms of VacA often lack the cag PAI [66]. The cag PAI encodes an effector protein, CagA, which causes numerous alterations in host cells, as well as a type IV secretion system required for entry of CagA into host cells. The co-selection of type s1 vacA and the cag PAI, as well as similarities in the phylogenetic structure of vacA and cagA genes [13], suggests the existence of a functional interaction between VacA and products of the cag PAI. In support of this hypothesis, several studies have shown that VacA partially inhibits the actions of CagA in vitro, and CagA partially inhibits the trafficking or actions of VacA [101,183,184,185,186,187]. Thus, another important action of VacA in vivo may be to provide an optimal counterbalance for the actions of CagA.
11. Summary
In summary, VacA is a secreted bacterial toxin that differs substantially from other known toxins in amino acid sequence, structure, intracellular trafficking, and actions. Many different cell types are susceptible to VacA in vitro, and the toxin can cause a wide spectrum of cellular alterations. In vivo, VacA enhances the capacity of H. pylori to colonize the stomach and contributes to the pathogenesis of H. pylori-induced diseases. In future studies, it will be important to define more clearly the mechanisms by which VacA causes alterations in host cells and to define the actions of this toxin that are most relevant in vivo.
Acknowledgments
Supported by NIH AI039657, CA116087, AI118932, T32 GM008320, and Department of Veterans Affairs Merit Review grant 2I01BX000627.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Marshall B.J., Warren J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Leunk R.D., Johnson P.T., David B.C., Kraft W.G., Morgan D.R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J. Med. Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- 3.Cover T.L., Blaser M.J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J. Biol. Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 4.Cover T.L., Tummuru M.K.R., Cao P., Thompson S.A., Blaser M.J. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J. Biol. Chem. 1994;269:10566–10573. [PubMed] [Google Scholar]
- 5.Schmitt W., Haas R. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: Structural similarities with the IgA protease type of exported protein. Mol. Microbiol. 1994;12:307–319. doi: 10.1111/j.1365-2958.1994.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 6.Telford J.L., Ghiara P., Dell’Orco M., Comanducci M., Burroni D., Bugnoli M., Tecce M.F., Censini S., Covacci A., Xiang Z., et al. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J. Exp. Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phadnis S.H., Ilver D., Janzon L., Normark S., Westblom T.U. Pathological significance and molecular characterization of the vacuolating toxin gene of Helicobacter pylori. Infect. Immun. 1994;62:1557–1565. doi: 10.1128/iai.62.5.1557-1565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kersulyte D., Rossi M., Berg D.E. Sequence divergence and conservation in genomes of Helicobacter cetorum strains from a dolphin and a whale. PLoS ONE. 2013;8:173. doi: 10.1371/journal.pone.0083177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harper C.G., Feng Y., Xu S., Taylor N.S., Kinsel M., Dewhirst F.E., Paster B.J., Greenwell M., Levine G., Rogers A., et al. Helicobacter cetorum sp. nov., a urease-positive helicobacter species isolated from dolphins and whales. J. Clin. Microbiol. 2002;40:4536–4543. doi: 10.1128/JCM.40.12.4536-4543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman C.G., Loureiro J.D., Matteo M.J., Catalano M., Gonzalez A.B., Heredia S.R., Zubillaga M.B., Solnick J.V., Cremaschi G.A. Helicobacter spp. From gastric biopsies of stranded south american fur seals (Arctocephalus australis) Res. Vet. Sci. 2009;86:18–21. doi: 10.1016/j.rvsc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Dailidiene D., Dailide G., Ogura K., Zhang M., Mukhopadhyay A.K., Eaton K.A., Cattoli G., Kusters J.G., Berg D.E. Helicobacter acinonychis: Genetic and rodent infection studies of a Helicobacter pylori-like gastric pathogen of cheetahs and other big cats. J. Bacteriol. 2004;186:356–365. doi: 10.1128/JB.186.2.356-365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eppinger M., Baar C., Linz B., Raddatz G., Lanz C., Keller H., Morelli G., Gressmann H., Achtman M., Schuster S.C. Who ate whom? Adaptive helicobacter genomic changes that accompanied a host jump from early humans to large felines. PLoS Genet. 2006;2:173. doi: 10.1371/journal.pgen.0020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangwer K.A., Shaffer C.L., Suerbaum S., Lacy D.B., Cover T.L., Bordenstein S.R. Molecular evolution of the Helicobacter pylori vacuolating toxin gene vacA. J. Bacteriol. 2010;192:6126–6135. doi: 10.1128/JB.01081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer W., Buhrdorf R., Gerland E., Haas R. Outer membrane targeting of passenger proteins by the vacuolating cytotoxin autotransporter of Helicobacter pylori. Infect. Immun. 2001;69:6769–6775. doi: 10.1128/IAI.69.11.6769-6775.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sause W.E., Castillo A.R., Ottemann K.M. The Helicobacter pylori autotransporter ImaA (HP0289) modulates the immune response and contributes to host colonization. Infect. Immun. 2012;80:2286–2296. doi: 10.1128/IAI.00312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radin J.N., Gaddy J.A., Gonzalez-Rivera C., Loh J.T., Algood H.M., Cover T.L. Flagellar localization of a Helicobacter pylori autotransporter protein. MBio. 2013;4:e00613-12. doi: 10.1128/mBio.00613-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kavermann H., Burns B.P., Angermuller K., Odenbreit S., Fischer W., Melchers K., Haas R. Identification and characterization of Helicobacter pylori genes essential for gastric colonization. J. Exp. Med. 2003;197:813–822. doi: 10.1084/jem.20021531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldwin D.N., Shepherd B., Kraemer P., Hall M.K., Sycuro L.K., Pinto-Santini D.M., Salama N.R. Identification of Helicobacter pylori genes that contribute to stomach colonization. Infect. Immun. 2007;75:1005–1016. doi: 10.1128/IAI.01176-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castillo A.R., Woodruff A.J., Connolly L.E., Sause W.E., Ottemann K.M. Recombination-based in vivo expression technology identifies Helicobacter pylori genes important for host colonization. Infect. Immun. 2008;76:5632–5644. doi: 10.1128/IAI.00627-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voss B.J., Gaddy J.A., McDonald W.H., Cover T.L. Analysis of surface-exposed outer membrane proteins in Helicobacter pylori. J. Bacteriol. 2014;196:2455–2471. doi: 10.1128/JB.01768-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albert T.J., Dailidiene D., Dailide G., Norton J.E., Kalia A., Richmond T.A., Molla M., Singh J., Green R.D., Berg D.E. Mutation discovery in bacterial genomes: Metronidazole resistance in Helicobacter pylori. Nat. Methods. 2005;2:951–953. doi: 10.1038/nmeth805. [DOI] [PubMed] [Google Scholar]
- 22.Forsyth M.H., Cover T.L. Mutational analysis of the vacA promoter provides insight into gene transcription in Helicobacter pylori. J. Bacteriol. 1999;181:2261–2266. doi: 10.1128/jb.181.7.2261-2266.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amilon K.R., Letley D.P., Winter J.A., Robinson K., Atherton J.C. Expression of the Helicobacter pylori virulence factor vacuolating cytotoxin A (vacA) is influenced by a potential stem-loop structure in the 5′ untranslated region of the transcript. Mol. Microbiol. 2015;98:831–846. doi: 10.1111/mmi.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forsyth M.H., Cover T.L. Intercellular communication in Helicobacter pylori: LuxS is essential for the production of an extracellular signaling molecule. Infect. Immun. 2000;68:3193–3199. doi: 10.1128/IAI.68.6.3193-3199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snider C.A., Voss B.J., McDonald W.H., Cover T.L. Growth phase-dependent composition of the Helicobacter pylori exoproteome. J. Proteom. 2016;130:94–107. doi: 10.1016/j.jprot.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merrell D.S., Thompson L.J., Kim C.C., Mitchell H., Tompkins L.S., Lee A., Falkow S. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect. Immun. 2003;71:6510–6525. doi: 10.1128/IAI.71.11.6510-6525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Amsterdam K., van Vliet A.H., Kusters J.G., Feller M., Dankert J., van der Ende A. Induced Helicobacter pylori vacuolating cytotoxin VacA expression after initial colonisation of human gastric epithelial cells. FEMS Immunol. Med. Microbiol. 2003;39:251–256. doi: 10.1016/S0928-8244(03)00226-8. [DOI] [PubMed] [Google Scholar]
- 28.Gancz H., Jones K.R., Merrell D.S. Sodium chloride affects Helicobacter pylori growth and gene expression. J. Bacteriol. 2008;190:4100–4105. doi: 10.1128/JB.01728-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen V.Q., Caprioli R.M., Cover T.L. Carboxy-terminal proteolytic processing of Helicobacter pylori vacuolating toxin. Infect. Immun. 2001;69:543–546. doi: 10.1128/IAI.69.1.543-546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bumann D., Aksu S., Wendland M., Janek K., Zimny-Arndt U., Sabarth N., Meyer T.F., Jungblut P.R. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect. Immun. 2002;70:3396–3403. doi: 10.1128/IAI.70.7.3396-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilver D., Barone S., Mercati D., Lupetti P., Telford J.L. Helicobacter pylori toxin VacA is transferred to host cells via a novel contact-dependent mechanism. Cell. Microbiol. 2004;6:167–174. doi: 10.1046/j.1462-5822.2003.00349.x. [DOI] [PubMed] [Google Scholar]
- 32.Czajkowsky D.M., Iwamoto H., Cover T.L., Shao Z. The vacuolating toxin from Helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc. Natl. Acad. Sci. USA. 1999;96:2001–2006. doi: 10.1073/pnas.96.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tombola F., Carlesso C., Szabo I., de Bernard M., Reyrat J.M., Telford J.L., Rappuoli R., Montecucco C., Papini E., Zoratti M. Helicobacter pylori vacuolating toxin forms anion-selective channels in planar lipid bilayers: Possible implications for the mechanism of cellular vacuolation. Biophys. J. 1999;76:1401–1409. doi: 10.1016/S0006-3495(99)77301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwamoto H., Czajkowsky D.M., Cover T.L., Szabo G., Shao Z. VacA from Helicobacter pylori: A hexameric chloride channel. FEBS Lett. 1999;450:101–104. doi: 10.1016/S0014-5793(99)00474-3. [DOI] [PubMed] [Google Scholar]
- 35.Tombola F., Oregna F., Brutsche S., Szabo I., Del Giudice G., Rappuoli R., Montecucco C., Papini E., Zoratti M. Inhibition of the vacuolating and anion channel activities of the VacA toxin of Helicobacter pylori. FEBS Lett. 1999;460:221–225. doi: 10.1016/S0014-5793(99)01348-4. [DOI] [PubMed] [Google Scholar]
- 36.Szabo I., Brutsche S., Tombola F., Moschioni M., Satin B., Telford J.L., Rappuoli R., Montecucco C., Papini E., Zoratti M. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. Embo J. 1999;18:5517–5527. doi: 10.1093/emboj/18.20.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Czajkowsky D.M., Iwamoto H., Szabo G., Cover T.L., Shao Z. Mimicry of a host anion channel by a Helicobacter pylori pore-forming toxin. Biophys. J. 2005;89:3093–3101. doi: 10.1529/biophysj.105.066746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vinion-Dubiel A.D., McClain M.S., Czajkowsky D.M., Iwamoto H., Ye D., Cao P., Schraw W., Szabo G., Blanke S.R., Shao Z., et al. A dominant negative mutant of Helicobacter pylori vacuolating toxin (VacA) inhibits VacA-induced cell vacuolation. J. Biol. Chem. 1999;274:37736–37742. doi: 10.1074/jbc.274.53.37736. [DOI] [PubMed] [Google Scholar]
- 39.McClain M.S., Iwamoto H., Cao P., Vinion-Dubiel A.D., Li Y., Szabo G., Shao Z., Cover T.L. Essential role of a GXXXG motif for membrane channel formation by Helicobacter pylori vacuolating toxin. J. Biol. Chem. 2003;278:12101–12108. doi: 10.1074/jbc.M212595200. [DOI] [PubMed] [Google Scholar]
- 40.Torres V.J., McClain M.S., Cover T.L. Interactions between p-33 and p-55 domains of the Helicobacter pylori vacuolating cytotoxin (VacA) J. Biol. Chem. 2004;279:2324–2331. doi: 10.1074/jbc.M310159200. [DOI] [PubMed] [Google Scholar]
- 41.Torres V.J., Ivie S.E., McClain M.S., Cover T.L. Functional properties of the p33 and p55 domains of the Helicobacter pylori vacuolating cytotoxin. J. Biol. Chem. 2005;280:21107–21114. doi: 10.1074/jbc.M501042200. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Rivera C., Gangwer K.A., McClain M.S., Eli I.M., Chambers M.G., Ohi M.D., Lacy D.B., Cover T.L. Reconstitution of Helicobacter pylori VacA toxin from purified components. Biochemistry. 2010;49:5743–5752. doi: 10.1021/bi100618g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gangwer K.A., Mushrush D.J., Stauff D.L., Spiller B., McClain M.S., Cover T.L., Lacy D.B. Crystal structure of the Helicobacter pylori vacuolating toxin p55 domain. Proc. Natl. Acad. Sci. USA. 2007;104:16293–16298. doi: 10.1073/pnas.0707447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Junker M., Schuster C.C., McDonnell A.V., Sorg K.A., Finn M.C., Berger B., Clark P.L. Pertactin beta-helix folding mechanism suggests common themes for the secretion and folding of autotransporter proteins. Proc. Natl. Acad. Sci. USA. 2006;103:4918–4923. doi: 10.1073/pnas.0507923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Bernard M., Arico B., Papini E., Rizzuto R., Grandi G., Rappuoli R., Montecucco C. Helicobacter pylori toxin VacA induces vacuole formation by acting in the cell cytosol. Mol. Microbiol. 1997;26:665–674. doi: 10.1046/j.1365-2958.1997.5881952.x. [DOI] [PubMed] [Google Scholar]
- 46.De Bernard M., Burroni D., Papini E., Rappuoli R., Telford J., Montecucco C. Identification of the Helicobacter pylori VacA toxin domain active in the cell cytosol. Infect. Immun. 1998;66:6014–6016. doi: 10.1128/iai.66.12.6014-6016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye D., Willhite D.C., Blanke S.R. Identification of the minimal intracellular vacuolating domain of the Helicobacter pylori vacuolating toxin. J. Biol. Chem. 1999;274:9277–9282. doi: 10.1074/jbc.274.14.9277. [DOI] [PubMed] [Google Scholar]
- 48.Kim S., Chamberlain A.K., Bowie J.U. Membrane channel structure of Helicobacter pylori vacuolating toxin: Role of multiple GXXXG motifs in cylindrical channels. Proc. Natl. Acad. Sci. USA. 2004;101:5988–5991. doi: 10.1073/pnas.0308694101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McClain M.S., Cao P., Cover T.L. Amino-terminal hydrophobic region of Helicobacter pylori vacuolating cytotoxin (VacA) mediates transmembrane protein dimerization. Infect. Immun. 2001;69:1181–1184. doi: 10.1128/IAI.69.2.1181-1184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye D., Blanke S.R. Mutational analysis of the Helicobacter pylori vacuolating toxin amino terminus: Identification of amino acids essential for cellular vacuolation. Infect. Immun. 2000;68:4354–4357. doi: 10.1128/IAI.68.7.4354-4357.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McClain M.S., Czajkowsky D.M., Torres V.J., Szabo G., Shao Z., Cover T.L. Random mutagenesis of Helicobacter pylori VacA to identify amino acids essential for vacuolating cytotoxic activity. Infect. Immun. 2006;74:6188–6195. doi: 10.1128/IAI.00915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lupetti P., Heuser J.E., Manetti R., Massari P., Lanzavecchia S., Bellon P.L., Dallai R., Rappuoli R., Telford J.L. Oligomeric and subunit structure of the Helicobacter pylori vacuolating cytotoxin. J. Cell Biol. 1996;133:801–807. doi: 10.1083/jcb.133.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Bez C., Adrian M., Dubochet J., Cover T.L. High resolution structural analysis of Helicobacter pylori VacA toxin oligomers by cryo-negative staining electron microscopy. J. Struct. Biol. 2005;151:215–228. doi: 10.1016/j.jsb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Chambers M.G., Pyburn T.M., Gonzalez-Rivera C., Collier S.E., Eli I., Yip C.K., Takizawa Y., Lacy D.B., Cover T.L., Ohi M.D. Structural analysis of the oligomeric states of Helicobacter pylori VacA toxin. J. Mol. Biol. 2013;425:524–535. doi: 10.1016/j.jmb.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye D., Blanke S.R. Functional complementation reveals the importance of intermolecular monomer interactions for Helicobacter pylori VacA vacuolating activity. Mol. Microbiol. 2002;43:1243–1253. doi: 10.1046/j.1365-2958.2002.02818.x. [DOI] [PubMed] [Google Scholar]
- 56.Willhite D.C., Ye D., Blanke S.R. Fluorescence resonance energy transfer microscopy of the Helicobacter pylori vacuolating cytotoxin within mammalian cells. Infect. Immun. 2002;70:3824–3832. doi: 10.1128/IAI.70.7.3824-3832.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Genisset C., Galeotti C.L., Lupetti P., Mercati D., Skibinski D.A., Barone S., Battistutta R., de Bernard M., Telford J.L. A Helicobacter pylori vacuolating toxin mutant that fails to oligomerize has a dominant negative phenotype. Infect. Immun. 2006;74:1786–1794. doi: 10.1128/IAI.74.3.1786-1794.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ivie S.E., McClain M.S., Torres V.J., Algood H.M., Lacy D.B., Yang R., Blanke S.R., Cover T.L. Helicobacter pylori VacA subdomain required for intracellular toxin activity and assembly of functional oligomeric complexes. Infect. Immun. 2008;76:2843–2851. doi: 10.1128/IAI.01664-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cover T.L., Hanson P.I., Heuser J.E. Acid-induced dissociation of VacA, the Helicobacter pylori vacuolating cytotoxin, reveals its pattern of assembly. J. Cell Biol. 1997;138:759–769. doi: 10.1083/jcb.138.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yahiro K., Niidome T., Kimura M., Hatakeyama T., Aoyagi H., Kurazono H., Imagawa K., Wada A., Moss J., Hirayama T. Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase beta. J. Biol. Chem. 1999;274:36693–36699. doi: 10.1074/jbc.274.51.36693. [DOI] [PubMed] [Google Scholar]
- 61.De Bernard M., Papini E., de Filippis V., Gottardi E., Telford J., Manetti R., Fontana A., Rappuoli R., Montecucco C. Low pH activates the vacuolating toxin of Helicobacter pylori, which becomes acid and pepsin resistant. J. Biol. Chem. 1995;270:23937–23940. doi: 10.1074/jbc.270.41.23937. [DOI] [PubMed] [Google Scholar]
- 62.McClain M.S., Schraw W., Ricci V., Boquet P., Cover T.L. Acid-activation of Helicobacter pylori vacuolating cytotoxin (VacA) results in toxin internalization by eukaryotic cells. Mol. Microbiol. 2000;37:433–442. doi: 10.1046/j.1365-2958.2000.02013.x. [DOI] [PubMed] [Google Scholar]
- 63.Forsyth M.H., Atherton J.C., Blaser M.J., Cover T.L. Heterogeneity in levels of vacuolating cytotoxin gene (vacA) transcription among Helicobacter pylori strains. Infect. Immun. 1998;66:3088–3094. doi: 10.1128/iai.66.7.3088-3094.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ito Y., Azuma T., Ito S., Suto H., Miyaji H., Yamazaki Y., Kohli Y., Kuriyama M. Full-length sequence analysis of the vacA gene from cytotoxic and noncytotoxic Helicobacter pylori. J. Infect. Dis. 1998;178:1391–1398. doi: 10.1086/314435. [DOI] [PubMed] [Google Scholar]
- 65.Duncan S.S., Valk P.L., McClain M.S., Shaffer C.L., Metcalf J.A., Bordenstein S.R., Cover T.L. Comparative genomic analysis of east asian and non-asian Helicobacter pylori strains identifies rapidly evolving genes. PLoS ONE. 2013;8:173. doi: 10.1371/journal.pone.0055120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atherton J.C., Cao P., Peek R.M., Jr., Tummuru M.K., Blaser M.J., Cover T.L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 67.Rhead J.L., Letley D.P., Mohammadi M., Hussein N., Mohagheghi M.A., Eshagh Hosseini M., Atherton J.C. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926–936. doi: 10.1053/j.gastro.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 68.Letley D.P., Atherton J.C. Natural diversity in the n terminus of the mature vacuolating cytotoxin of Helicobacter pylori determines cytotoxin activity. J. Bacteriol. 2000;182:3278–3280. doi: 10.1128/JB.182.11.3278-3280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McClain M.S., Cao P., Iwamoto H., Vinion-Dubiel A.D., Szabo G., Shao Z., Cover T.L. A 12-amino-acid segment, present in type s2 but not type s1 Helicobacter pylori VacA proteins, abolishes cytotoxin activity and alters membrane channel formation. J. Bacteriol. 2001;183:6499–6508. doi: 10.1128/JB.183.22.6499-6508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Letley D.P., Rhead J.L., Twells R.J., Dove B., Atherton J.C. Determinants of non-toxicity in the gastric pathogen Helicobacter pylori. J. Biol. Chem. 2003;278:26734–26741. doi: 10.1074/jbc.M304071200. [DOI] [PubMed] [Google Scholar]
- 71.Pagliaccia C., de Bernard M., Lupetti P., Ji X., Burroni D., Cover T.L., Papini E., Rappuoli R., Telford J.L., Reyrat J.M. The m2 form of the Helicobacter pylori cytotoxin has cell type-specific vacuolating activity. Proc. Natl. Acad. Sci. USA. 1998;95:10212–10217. doi: 10.1073/pnas.95.17.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ji X., Fernandez T., Burroni D., Pagliaccia C., Atherton J.C., Reyrat J.M., Rappuoli R., Telford J.L. Cell specificity of Helicobacter pylori cytotoxin is determined by a short region in the polymorphic midregion. Infect. Immun. 2000;68:3754–3757. doi: 10.1128/IAI.68.6.3754-3757.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tombola F., Pagliaccia C., Campello S., Telford J.L., Montecucco C., Papini E., Zoratti M. How the loop and middle regions influence the properties of Helicobacter pylori VacA channels. Biophys. J. 2001;81:3204–3215. doi: 10.1016/S0006-3495(01)75956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gonzalez-Rivera C., Algood H.M., Radin J.N., McClain M.S., Cover T.L. The intermediate region of Helicobacter pylori VacA is a determinant of toxin potency in a jurkat t cell assay. Infect. Immun. 2012;80:2578–2588. doi: 10.1128/IAI.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Figueiredo C., Machado J.C., Pharoah P., Seruca R., Sousa S., Carvalho R., Capelinha A.F., Quint W., Caldas C., van Doorn L.J., et al. Helicobacter pylori and interleukin 1 genotyping: An opportunity to identify high-risk individuals for gastric carcinoma. J. Natl. Cancer Inst. 2002;94:1680–1687. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- 76.Cover T.L. Helicobacter pylori diversity and gastric cancer risk. MBio. 2016;7:e01869-15. doi: 10.1128/mBio.01869-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cover T.L., Vaughn S.G., Cao P., Blaser M.J. Potentiation of Helicobacter pylori vacuolating toxin activity by nicotine and other weak bases. J. Infect. Dis. 1992;166:1073–1078. doi: 10.1093/infdis/166.5.1073. [DOI] [PubMed] [Google Scholar]
- 78.Papini E., de Bernard M., Milia E., Bugnoli M., Zerial M., Rappuoli R., Montecucco C. Cellular vacuoles induced by Helicobacter pylori originate from late endosomal compartments. Proc. Natl. Acad. Sci. USA. 1994;91:9720–9724. doi: 10.1073/pnas.91.21.9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Molinari M., Galli C., Norais N., Telford J.L., Rappuoli R., Luzio J.P., Montecucco C. Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers. J. Biol. Chem. 1997;272:25339–25344. doi: 10.1074/jbc.272.40.25339. [DOI] [PubMed] [Google Scholar]
- 80.Li Y., Wandinger-Ness A., Goldenring J.R., Cover T.L. Clustering and redistribution of late endocytic compartments in response to Helicobacter pylori vacuolating toxin. Mol. Biol. Cell. 2004;15:1946–1959. doi: 10.1091/mbc.E03-08-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kern B., Jain U., Utsch C., Otto A., Busch B., Jimenez-Soto L., Becher D., Haas R. Characterization of Helicobacter pylori VacA-containing vacuoles (VCVs), VacA intracellular trafficking and interference with calcium signalling in T lymphocytes. Cell. Microbiol. 2015;17:1811–1832. doi: 10.1111/cmi.12474. [DOI] [PubMed] [Google Scholar]
- 82.De Bernard M., Moschioni M., Habermann A., Griffiths G., Montecucco C. Cell vacuolization induced by Helicobacter pylori VacA cytotoxin does not depend on late endosomal snares. Cell. Microbiol. 2002;4:11–18. doi: 10.1046/j.1462-5822.2002.00163.x. [DOI] [PubMed] [Google Scholar]
- 83.Montecucco C., Rappuoli R. Living dangerously: How Helicobacter pylori survives in the human stomach. Nat. Rev. Mol. Cell Biol. 2001;2:457–466. doi: 10.1038/35073084. [DOI] [PubMed] [Google Scholar]
- 84.Cover T.L., Blanke S.R. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Microbiol. 2005;3:320–332. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 85.Ricci V., Sommi P., Fiocca R., Romano M., Solcia E., Ventura U. Helicobacter pylori vacuolating toxin accumulates within the endosomal-vacuolar compartment of cultured gastric cells and potentiates the vacuolating activity of ammonia. J. Pathol. 1997;183:453–459. doi: 10.1002/(SICI)1096-9896(199712)183:4<453::AID-PATH950>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 86.Morbiato L., Tombola F., Campello S., Del Giudice G., Rappuoli R., Zoratti M., Papini E. Vacuolation induced by VacA toxin of Helicobacter pylori requires the intracellular accumulation of membrane permeant bases, Cl(-) and water. FEBS Lett. 2001;508:479–483. doi: 10.1016/S0014-5793(01)03133-7. [DOI] [PubMed] [Google Scholar]
- 87.Genisset C., Puhar A., Calore F., de Bernard M., Dell’Antone P., Montecucco C. The concerted action of the Helicobacter pylori cytotoxin VacA and of the v-atpase proton pump induces swelling of isolated endosomes. Cell. Microbiol. 2007;9:1481–1490. doi: 10.1111/j.1462-5822.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 88.Satin B., Norais N., Telford J., Rappuoli R., Murgia M., Montecucco C., Papini E. Effect of Helicobacter pylori vacuolating toxin on maturation and extracellular release of procathepsin d and on epidermal growth factor degradation. J. Biol. Chem. 1997;272:25022–25028. doi: 10.1074/jbc.272.40.25022. [DOI] [PubMed] [Google Scholar]
- 89.Tan S., Noto J.M., Romero-Gallo J., Peek R.M., Jr., Amieva M.R. Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS Pathog. 2011;7:173. doi: 10.1371/journal.ppat.1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molinari M., Salio M., Galli C., Norais N., Rappuoli R., Lanzavecchia A., Montecucco C. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J. Exp. Med. 1998;187:135–140. doi: 10.1084/jem.187.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Terebiznik M.R., Raju D., Vazquez C.L., Torbricki K., Kulkarni R., Blanke S.R., Yoshimori T., Colombo M.I., Jones N.L. Effect of Helicobacter pylori’s vacuolating cytotoxin on the autophagy pathway in gastric epithelial cells. Autophagy. 2009;5:370–379. doi: 10.4161/auto.5.3.7663. [DOI] [PubMed] [Google Scholar]
- 92.Yahiro K., Satoh M., Nakano M., Hisatsune J., Isomoto H., Sap J., Suzuki H., Nomura F., Noda M., Moss J., et al. Low-density lipoprotein receptor-related protein-1 (LRP1) mediates autophagy and apoptosis caused by Helicobacter pylori VacA. J. Biol. Chem. 2012;287:31104–31115. doi: 10.1074/jbc.M112.387498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raju D., Hussey S., Ang M., Terebiznik M.R., Sibony M., Galindo-Mata E., Gupta V., Blanke S.R., Delgado A., Romero-Gallo J., et al. Vacuolating cytotoxin and variants in Atg16l1 that disrupt autophagy promote Helicobacter pylori infection in humans. Gastroenterology. 2012;142:1160–1171. doi: 10.1053/j.gastro.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Greenfield L.K., Jones N.L. Modulation of autophagy by Helicobacter pylori and its role in gastric carcinogenesis. Trends Microbiol. 2013;21:602–612. doi: 10.1016/j.tim.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 95.Kimura M., Goto S., Wada A., Yahiro K., Niidome T., Hatakeyama T., Aoyagi H., Hirayama T., Kondo T. Vacuolating cytotoxin purified from Helicobacter pylori causes mitochondrial damage in human gastric cells. Microb. Pathog. 1999;26:45–52. doi: 10.1006/mpat.1998.0241. [DOI] [PubMed] [Google Scholar]
- 96.Willhite D.C., Blanke S.R. Helicobacter pylori vacuolating cytotoxin enters cells, localizes to the mitochondria, and induces mitochondrial membrane permeability changes correlated to toxin channel activity. Cell. Microbiol. 2004;6:143–154. doi: 10.1046/j.1462-5822.2003.00347.x. [DOI] [PubMed] [Google Scholar]
- 97.Yamasaki E., Wada A., Kumatori A., Nakagawa I., Funao J., Nakayama M., Hisatsune J., Kimura M., Moss J., Hirayama T. Helicobacter pylori vacuolating cytotoxin induces activation of the proapoptotic proteins Bax and Bak, leading to cytochrome c release and cell death, independent of vacuolation. J. Biol. Chem. 2006;281:11250–11259. doi: 10.1074/jbc.M509404200. [DOI] [PubMed] [Google Scholar]
- 98.Galmiche A., Rassow J., Doye A., Cagnol S., Chambard J.C., Contamin S., de Thillot V., Just I., Ricci V., Solcia E., et al. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. Embo J. 2000;19:6361–6370. doi: 10.1093/emboj/19.23.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Willhite D.C., Cover T.L., Blanke S.R. Cellular vacuolation and mitochondrial cytochrome c release are independent outcomes of Helicobacter pylori vacuolating cytotoxin activity that are each dependent on membrane channel formation. J. Biol. Chem. 2003;278:48204–48209. doi: 10.1074/jbc.M304131200. [DOI] [PubMed] [Google Scholar]
- 100.Jain P., Luo Z.Q., Blanke S.R. Helicobacter pylori vacuolating cytotoxin a (VacA) engages the mitochondrial fission machinery to induce host cell death. Proc. Natl. Acad. Sci. USA. 2011;108:16032–16037. doi: 10.1073/pnas.1105175108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oldani A., Cormont M., Hofman V., Chiozzi V., Oregioni O., Canonici A., Sciullo A., Sommi P., Fabbri A., Ricci V., et al. Helicobacter pylori counteracts the apoptotic action of its VacA toxin by injecting the CagA protein into gastric epithelial cells. PLoS Pathog. 2009;5:173. doi: 10.1371/journal.ppat.1000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Domanska G., Motz C., Meinecke M., Harsman A., Papatheodorou P., Reljic B., Dian-Lothrop E.A., Galmiche A., Kepp O., Becker L., et al. Helicobacter pylori VacA toxin/subunit p34: Targeting of an anion channel to the inner mitochondrial membrane. PLoS Pathog. 2010;6:173. doi: 10.1371/journal.ppat.1000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Foo J.H., Culvenor J.G., Ferrero R.L., Kwok T., Lithgow T., Gabriel K. Both the p33 and p55 subunits of the Helicobacter pylori VacA toxin are targeted to mammalian mitochondria. J. Mol. Biol. 2010;401:792–798. doi: 10.1016/j.jmb.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 104.Tombola F., Morbiato L., Del Giudice G., Rappuoli R., Zoratti M., Papini E. The Helicobacter pylori VacA toxin is a urea permease that promotes urea diffusion across epithelia. J. Clin. Investig. 2001;108:929–937. doi: 10.1172/JCI13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Debellis L., Papini E., Caroppo R., Montecucco C., Curci S. Helicobacter pylori cytotoxin VacA increases alkaline secretion in gastric epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G1440–G1448. doi: 10.1152/ajpgi.2001.281.6.G1440. [DOI] [PubMed] [Google Scholar]
- 106.Papini E., Satin B., Norais N., de Bernard M., Telford J.L., Rappuoli R., Montecucco C. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J. Clin. Investig. 1998;102:813–820. doi: 10.1172/JCI2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Amieva M.R., Vogelmann R., Covacci A., Tompkins L.S., Nelson W.J., Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pelicic V., Reyrat J.M., Sartori L., Pagliaccia C., Rappuoli R., Telford J.L., Montecucco C., Papini E. Helicobacter pylori VacA cytotoxin associated with the bacteria increases epithelial permeability independently of its vacuolating activity. Microbiology. 1999;145:2043–2050. doi: 10.1099/13500872-145-8-2043. [DOI] [PubMed] [Google Scholar]
- 109.Nakayama M., Kimura M., Wada A., Yahiro K., Ogushi K.I., Niidome T., Fujikawa A., Shirasaka D., Aoyama N., Kurazono H., et al. Helicobacter pylori VacA activates the p38/ATF-2-mediated signal pathway in AZ-521 cells. J. Biol. Chem. 2004;279:7024–7028. doi: 10.1074/jbc.M308898200. [DOI] [PubMed] [Google Scholar]
- 110.Hisatsune J., Yamasaki E., Nakayama M., Shirasaka D., Kurazono H., Katagata Y., Inoue H., Han J., Sap J., Yahiro K., et al. Helicobacter pylori VacA enhances PGE2 production through induction of COX-2 expression via a p38 MAP kinase/ATF-2 cascade in AZ-521 cells. Infect. Immun. 2007;75:4472–4481. doi: 10.1128/IAI.00500-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boncristiano M., Paccani S.R., Barone S., Ulivieri C., Patrussi L., Ilver D., Amedei A., D’Elios M.M., Telford J.L., Baldari C.T. The Helicobacter pylori vacuolating toxin inhibits T cell activation by two independent mechanisms. J. Exp. Med. 2003;198:1887–1897. doi: 10.1084/jem.20030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fujikawa A., Shirasaka D., Yamamoto S., Ota H., Yahiro K., Fukada M., Shintani T., Wada A., Aoyama N., Hirayama T., et al. Mice deficient in protein tyrosine phosphatase receptor type z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat. Genet. 2003;33:375–381. doi: 10.1038/ng1112. [DOI] [PubMed] [Google Scholar]
- 113.Caputo R., Tuccillo C., Manzo B.A., Zarrilli R., Tortora G., Blanco Cdel V., Ricci V., Ciardiello F., Romano M. Helicobacter pylori VacA toxin up-regulates vascular endothelial growth factor expression in MKN 28 gastric cells through an epidermal growth factor receptor-, cyclooxygenase-2-dependent mechanism. Clin. Cancer Res. 2003;9:2015–2021. [PubMed] [Google Scholar]
- 114.Nakayama M., Hisatsune J., Yamasaki E., Isomoto H., Kurazono H., Hatakeyama M., Azuma T., Yamaoka Y., Yahiro K., Moss J., et al. Helicobacter pylori VacA-induced inhibition of GSK3 through the PI3K/Akt signaling pathway. J. Biol. Chem. 2009;284:1612–1619. doi: 10.1074/jbc.M806981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuck D., Kolmerer B., Iking-Konert C., Krammer P.H., Stremmel W., Rudi J. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line ags. Infect. Immun. 2001;69:5080–5087. doi: 10.1128/IAI.69.8.5080-5087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cover T.L., Krishna U.S., Israel D.A., Peek R.M., Jr. Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 2003;63:951–957. [PubMed] [Google Scholar]
- 117.Radin J.N., Gonzalez-Rivera C., Ivie S.E., McClain M.S., Cover T.L. Helicobacter pylori VacA induces programmed necrosis in gastric epithelial cells. Infect. Immun. 2011;79:2535–2543. doi: 10.1128/IAI.01370-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Radin J.N., Gonzalez-Rivera C., Frick-Cheng A.E., Sheng J., Gaddy J.A., Rubin D.H., Algood H.M., McClain M.S., Cover T.L. Role of connexin 43 in Helicobacter pylori VacA-induced cell death. Infect. Immun. 2014;82:423–432. doi: 10.1128/IAI.00827-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Calore F., Genisset C., Casellato A., Rossato M., Codolo G., Esposti M.D., Scorrano L., de Bernard M. Endosome-mitochondria juxtaposition during apoptosis induced by H. pylori VacA. Cell Death Differ. 2010;17:1707–1716. doi: 10.1038/cdd.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matsumoto A., Isomoto H., Nakayama M., Hisatsune J., Nishi Y., Nakashima Y., Matsushima K., Kurazono H., Nakao K., Hirayama T., et al. Helicobacter pylori VacA reduces the cellular expression of STAT3 and pro-survival Bcl-2 family proteins, Bcl-2 and Bcl-XL, leading to apoptosis in gastric epithelial cells. Dig. Dis. Sci. 2011;56:999–1006. doi: 10.1007/s10620-010-1420-1. [DOI] [PubMed] [Google Scholar]
- 121.Akazawa Y., Isomoto H., Matsushima K., Kanda T., Minami H., Yamaghchi N., Taura N., Shiozawa K., Ohnita K., Takeshima F., et al. Endoplasmic reticulum stress contributes to Helicobacter pylori VacA-induced apoptosis. PLoS ONE. 2013;8:173. doi: 10.1371/journal.pone.0082322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim I.J., Blanke S.R. Remodeling the host environment: Modulation of the gastric epithelium by the Helicobacter pylori vacuolating toxin (VacA) Front. Cell. Infect. Microbiol. 2012;2:37. doi: 10.3389/fcimb.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim J.M., Kim J.S., Lee J.Y., Kim Y.J., Youn H.J., Kim I.Y., Chee Y.J., Oh Y.K., Kim N., Jung H.C., et al. Vacuolating cytotoxin in Helicobacter pylori water-soluble proteins upregulates chemokine expression in human eosinophils via Ca2+ influx, mitochondrial reactive oxygen intermediates, and NF-kappaB activation. Infect. Immun. 2007;75:3373–3381. doi: 10.1128/IAI.01940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim J.M., Kim J.S., Lee J.Y., Sim Y.S., Kim Y.J., Oh Y.K., Yoon H.J., Kang J.S., Youn J., Kim N., et al. Dual effects of Helicobacter pylori vacuolating cytotoxin on human eosinophil apoptosis in early and late periods of stimulation. Eur. J. Immunol. 2010;40:1651–1662. doi: 10.1002/eji.200939882. [DOI] [PubMed] [Google Scholar]
- 125.Supajatura V., Ushio H., Wada A., Yahiro K., Okumura K., Ogawa H., Hirayama T., Ra C. Cutting edge: VacA, a vacuolating cytotoxin of Helicobacter pylori, directly activates mast cells for migration and production of proinflammatory cytokines. J. Immunol. 2002;168:2603–2607. doi: 10.4049/jimmunol.168.6.2603. [DOI] [PubMed] [Google Scholar]
- 126.De Bernard M., Cappon A., Pancotto L., Ruggiero P., Rivera J., Del Giudice G., Montecucco C. The Helicobacter pylori VacA cytotoxin activates RBL-2H3 cells by inducing cytosolic calcium oscillations. Cell. Microbiol. 2005;7:191–198. doi: 10.1111/j.1462-5822.2004.00446.x. [DOI] [PubMed] [Google Scholar]
- 127.Kim J.M., Kim J.S., Yoo D.Y., Ko S.H., Kim N., Kim H., Kim Y.J. Stimulation of dendritic cells with Helicobacter pylori vacuolating cytotoxin negatively regulates their maturation via the restoration of E2F1. Clin. Exp. Immunol. 2011;166:34–45. doi: 10.1111/j.1365-2249.2011.04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oertli M., Noben M., Engler D.B., Semper R.P., Reuter S., Maxeiner J., Gerhard M., Taube C., Muller A. Helicobacter pylori gamma-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc. Natl. Acad. Sci. USA. 2013;110:3047–3052. doi: 10.1073/pnas.1211248110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gebert B., Fischer W., Weiss E., Hoffman R., Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301:1099–1102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- 130.Sundrud M.S., Torres V.J., Unutmaz D., Cover T.L. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc. Natl. Acad. Sci. USA. 2004;101:7727–7732. doi: 10.1073/pnas.0401528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Torres V.J., VanCompernolle S.E., Sundrud M.S., Unutmaz D., Cover T.L. Helicobacter pylori vacuolating cytotoxin inhibits activation-induced proliferation of human T and B lymphocyte subsets. J. Immunol. 2007;179:5433–5440. doi: 10.4049/jimmunol.179.8.5433. [DOI] [PubMed] [Google Scholar]
- 132.Allen L.A., Schlesinger L.S., Kang B. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 2000;191:115–128. doi: 10.1084/jem.191.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zheng P.Y., Jones N.L. Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell. Microbiol. 2003;5:25–40. doi: 10.1046/j.1462-5822.2003.00250.x. [DOI] [PubMed] [Google Scholar]
- 134.Weiss G., Forster S., Irving A., Tate M., Ferrero R.L., Hertzog P., Frokiaer H., Kaparakis-Liaskos M. Helicobacter pylori VacA suppresses lactobacillus acidophilus-induced interferon beta signaling in macrophages via alterations in the endocytic pathway. MBio. 2013;4:e00609-12. doi: 10.1128/mBio.00609-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hisatsune J., Nakayama M., Isomoto H., Kurazono H., Mukaida N., Mukhopadhyay A.K., Azuma T., Yamaoka Y., Sap J., Yamasaki E., et al. Molecular characterization of Helicobacter pylori VacA induction of IL-8 in U937 cells reveals a prominent role for p38 MAPK in activating transcription factor-2, cAMP response element binding protein, and NF-kappaB activation. J. Immunol. 2008;180:5017–5027. doi: 10.4049/jimmunol.180.7.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Menaker R.J., Ceponis P.J., Jones N.L. Helicobacter pylori induces apoptosis of macrophages in association with alterations in the mitochondrial pathway. Infect. Immun. 2004;72:2889–2898. doi: 10.1128/IAI.72.5.2889-2898.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kobayashi H., Kamiya S., Suzuki T., Kohda K., Muramatsu S., Kurumada T., Ohta U., Miyazawa M., Kimura N., Mutoh N., et al. The effect of Helicobacter pylori on gastric acid secretion by isolated parietal cells from a guinea pig. Association with production of vacuolating toxin by H. pylori. Scand. J. Gastroenterol. 1996;31:428–433. doi: 10.3109/00365529609006760. [DOI] [PubMed] [Google Scholar]
- 138.Wang F., Xia P., Wu F., Wang D., Wang W., Ward T., Liu Y., Aikhionbare F., Guo Z., Powell M., et al. Helicobacter pylori VacA disrupts apical membrane-cytoskeletal interactions in gastric parietal cells. J. Biol. Chem. 2008;283:26714–26725. doi: 10.1074/jbc.M800527200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Massari P., Manetti R., Burroni D., Nuti S., Norais N., Rappuoli R., Telford J.L. Binding of the Helicobacter pylori vacuolating cytotoxin to target cells. Infect. Immun. 1998;66:3981–3984. doi: 10.1128/iai.66.8.3981-3984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang H.J., Wang W.C. Expression and binding analysis of GST-VacA fusions reveals that the C-terminal approximately 100-residue segment of exotoxin is crucial for binding in hela cells [in process citation] Biochem. Biophys. Res. Commun. 2000;278:449–454. doi: 10.1006/bbrc.2000.3820. [DOI] [PubMed] [Google Scholar]
- 141.Wang W.-C., Wang H.-J., Kuo C.-H. Two distinctive cell binding patterns by vacuolating toxin fused with glutathione S-transferase: One high-affinity m1-specific binding and the other lower-affinity binding for variant m forms. Biochemistry. 2001;40:11887–11896. doi: 10.1021/bi010065u. [DOI] [PubMed] [Google Scholar]
- 142.Ricci V., Galmiche A., Doye A., Necchi V., Solcia E., Boquet P. High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis. Mol. Biol. Cell. 2000;11:3897–3909. doi: 10.1091/mbc.11.11.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Patel H.K., Willhite D.C., Patel R.M., Ye D., Williams C.L., Torres E.M., Marty K.B., MacDonald R.A., Blanke S.R. Plasma membrane cholesterol modulates cellular vacuolation induced by the Helicobacter pylori vacuolating cytotoxin. Infect. Immun. 2002;70:4112–4123. doi: 10.1128/IAI.70.8.4112-4123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yahiro K., Wada A., Nakayama M., Kimura T., Ogushi K., Niidome T., Aoyagi H., Yoshino K., Yonezawa K., Moss J., et al. Protein-tyrosine phosphatase alpha, RPTP alpha, is a Helicobacter pylori VacA receptor. J. Biol. Chem. 2003;278:19183–19189. doi: 10.1074/jbc.M300117200. [DOI] [PubMed] [Google Scholar]
- 145.Yahiro K., Wada A., Yamasaki E., Nakayama M., Nishi Y., Hisatsune J., Morinaga N., Sap J., Noda M., Moss J., et al. Essential domain of receptor tyrosine phosphatase beta (RPTPbeta) for interaction with Helicobacter pylori vacuolating cytotoxin. J. Biol. Chem. 2004;279:51013–51021. doi: 10.1074/jbc.M406473200. [DOI] [PubMed] [Google Scholar]
- 146.Seto K., Hayashi-Kuwabara Y., Yoneta T., Suda H., Tamaki H. Vacuolation induced by cytotoxin from Helicobacter pylori is mediated by the EGF receptor in hela cells. FEBS Lett. 1998;431:347–350. doi: 10.1016/S0014-5793(98)00788-1. [DOI] [PubMed] [Google Scholar]
- 147.Utt M., Danielsson B., Wadstrom T. Helicobacter pylori vacuolating cytotoxin binding to a putative cell surface receptor, heparan sulfate, studied by surface plasmon resonance. FEMS Immunol. Med. Microbiol. 2001;30:109–113. doi: 10.1111/j.1574-695X.2001.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 148.Gupta V.R., Patel H.K., Kostolansky S.S., Ballivian R.A., Eichberg J., Blanke S.R. Sphingomyelin functions as a novel receptor for Helicobacter pylori VacA. PLoS Pathog. 2008;4:173. doi: 10.1371/journal.ppat.1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gupta V.R., Wilson B.A., Blanke S.R. Sphingomyelin is important for the cellular entry and intracellular localization of Helicobacter pylori VacA. Cell. Microbiol. 2010;12:1517–1533. doi: 10.1111/j.1462-5822.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roche N., Ilver D., Angstrom J., Barone S., Telford J.L., Teneberg S. Human gastric glycosphingolipids recognized by Helicobacter pylori vacuolating cytotoxin VacA. Microbes Infect. 2007;9:605–614. doi: 10.1016/j.micinf.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 151.Molinari M., Galli C., de Bernard M., Norais N., Ruysschaert J.M., Rappuoli R., Montecucco C. The acid activation of Helicobacter pylori toxin VacA: Structural and membrane binding studies. Biochem. Biophys. Res. Commun. 1998;248:334–340. doi: 10.1006/bbrc.1998.8808. [DOI] [PubMed] [Google Scholar]
- 152.Schraw W., Li Y., McClain M.S., van der Goot F.G., Cover T.L. Association of Helicobacter pylori vacuolating toxin (VacA) with lipid rafts. J. Biol. Chem. 2002;277:34642–34650. doi: 10.1074/jbc.M203466200. [DOI] [PubMed] [Google Scholar]
- 153.Geisse N.A., Cover T.L., Henderson R.M., Edwardson J.M. Targeting of Helicobacter pylori vacuolating toxin to lipid raft membrane domains analysed by atomic force microscopy. Biochem. J. 2004;381:911–917. doi: 10.1042/BJ20031719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sewald X., Gebert-Vogal B., Prassl S., Barwig I., Weiss E., Fabbri M., Osicka R., Schiemann M., Busch D.H., Semmrich M., et al. CD18 is the T-lymphocyte receptor of the Helicobacter pylori vacuolating cytotoxin. Cell Host Microbe. 2008;3:20–29. doi: 10.1016/j.chom.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 155.Gauthier N.C., Ricci V., Gounon P., Doye A., Tauc M., Poujeol P., Boquet P. Glycosylphosphatidylinositol-anchored proteins and actin cytoskeleton modulate chloride transport by channels formed by the Helicobacter pylori vacuolating cytotoxin VacA in Hela cells. J. Biol. Chem. 2004;279:9481–9489. doi: 10.1074/jbc.M312040200. [DOI] [PubMed] [Google Scholar]
- 156.Gauthier N.C., Monzo P., Kaddai V., Doye A., Ricci V., Boquet P. Helicobacter pylori VacA cytotoxin: A probe for a clathrin-independent and Cdc42-dependent pinocytic pathway routed to late endosomes. Mol. Biol. Cell. 2005;16:4852–4866. doi: 10.1091/mbc.E05-05-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gauthier N.C., Monzo P., Gonzalez T., Doye A., Oldani A., Gounon P., Ricci V., Cormont M., Boquet P. Early endosomes associated with dynamic f-actin structures are required for late trafficking of H. pylori VacA toxin. J. Cell Biol. 2007;177:343–354. doi: 10.1083/jcb.200609061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sewald X., Jimenez-Soto L., Haas R. Pkc-dependent endocytosis of the Helicobacter pylori vacuolating cytotoxin in primary t lymphocytes. Cell. Microbiol. 2011;13:482–496. doi: 10.1111/j.1462-5822.2010.01551.x. [DOI] [PubMed] [Google Scholar]
- 159.Eaton K.A., Cover T.L., Tummuru M.K., Blaser M.J., Krakowka S. Role of vacuolating cytotoxin in gastritis due to Helicobacter pylori in gnotobiotic piglets. Infect. Immun. 1997;65:3462–3464. doi: 10.1128/iai.65.8.3462-3464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wirth H.P., Beins M.H., Yang M., Tham K.T., Blaser M.J. Experimental infection of mongolian gerbils with wild-type and mutant Helicobacter pylori strains. Infect. Immun. 1998;66:4856–4866. doi: 10.1128/iai.66.10.4856-4866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ogura K., Maeda S., Nakao M., Watanabe T., Tada M., Kyutoku T., Yoshida H., Shiratori Y., Omata M. Virulence factors of Helicobacter pylori responsible for gastric diseases in mongolian gerbil. J. Exp. Med. 2000;192:1601–1610. doi: 10.1084/jem.192.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Salama N.R., Otto G., Tompkins L., Falkow S. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect. Immun. 2001;69:730–736. doi: 10.1128/IAI.69.2.730-736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Winter J.A., Letley D.P., Cook K.W., Rhead J.L., Zaitoun A.A., Ingram R.J., Amilon K.R., Croxall N.J., Kaye P.V., Robinson K., et al. A role for the vacuolating cytotoxin, VacA, in colonization and Helicobacter pylori-induced metaplasia in the stomach. J. Infect. Dis. 2014;210:954–963. doi: 10.1093/infdis/jiu154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ghiara P., Marchetti M., Blaser M.J., Tummuru M.K., Cover T.L., Segal E.D., Tompkins L.S., Rappuoli R. Role of the Helicobacter pylori virulence factors vacuolating cytotoxin, CagA, and urease in a mouse model of disease. Infect. Immun. 1995;63:4154–4160. doi: 10.1128/iai.63.10.4154-4160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shirasaka D., Aoyama N., Sakashita M., Kuroda K., Maekawa S., Wambura C.M., Miyamoto M., Tamura T., Yahiro K., Wada A., et al. Relationship between gastric ulcer and Helicobacter pylori VacA detected in gastric juice using bead-ELISA method. Helicobacter. 2002;7:281–286. doi: 10.1046/j.1523-5378.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 166.Cover T.L., Cao P., Murthy U.K., Sipple M.S., Blaser M.J. Serum neutralizing antibody response to the vacuolating cytotoxin of Helicobacter pylori. J. Clin. Investig. 1992;90:913–918. doi: 10.1172/JCI115967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Perez-Perez G.I., Peek R.M., Jr., Atherton J.C., Blaser M.J., Cover T.L. Detection of anti-VacA antibody responses in serum and gastric juice samples using type s1/m1 and s2/m2 Helicobacter pylori VacA antigens. Clin. Diagn. Lab. Immunol. 1999;6:489–493. doi: 10.1128/cdli.6.4.489-493.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marchetti M., Aricò B., Burroni D., Figura N., Rappuoli R., Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 169.Ghiara P., Rossi M., Marchetti M., Di Tommaso A., Vindigni C., Ciampolini F., Covacci A., Telford J.L., De Magistris M.T., Pizza M., et al. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect. Immun. 1997;65:4996–5002. doi: 10.1128/iai.65.12.4996-5002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Marchetti M., Rossi M., Giannelli V., Giuliani M.M., Pizza M., Censini S., Covacci A., Massari P., Pagliaccia C., Manetti R., et al. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–37. doi: 10.1016/S0264-410X(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 171.Rossi G., Ruggiero P., Peppoloni S., Pancotto L., Fortuna D., Lauretti L., Volpini G., Mancianti S., Corazza M., Taccini E., et al. Therapeutic vaccination against Helicobacter pylori in the beagle dog experimental model: Safety, immunogenicity, and efficacy. Infect. Immun. 2004;72:3252–3259. doi: 10.1128/IAI.72.6.3252-3259.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Malfertheiner P., Schultze V., Rosenkranz B., Kaufmann S.H., Ulrichs T., Novicki D., Norelli F., Contorni M., Peppoloni S., Berti D., et al. Safety and immunogenicity of an intramuscular Helicobacter pylori vaccine in noninfected volunteers: A phase I study. Gastroenterology. 2008;135:787–795. doi: 10.1053/j.gastro.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 173.Liu K.Y., Shi Y., Luo P., Yu S., Chen L., Zhao Z., Mao X.H., Guo G., Wu C., Zou Q.M. Therapeutic efficacy of oral immunization with attenuated salmonella typhimurium expressing Helicobacter pylori CagA, VacA and UreB fusion proteins in mice model. Vaccine. 2011;29:6679–6685. doi: 10.1016/j.vaccine.2011.06.099. [DOI] [PubMed] [Google Scholar]
- 174.Cover T.L., Blaser M.J. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–1873. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Atherton J.C., Blaser M.J. Coadaptation of Helicobacter pylori and humans: Ancient history, modern implications. J. Clin. Investig. 2009;119:2475–2487. doi: 10.1172/JCI38605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Oertli M., Sundquist M., Hitzler I., Engler D.B., Arnold I.C., Reuter S., Maxeiner J., Hansson M., Taube C., Quiding-Jarbrink M., et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J. Clin. Investig. 2012;122:1082–1096. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Engler D.B., Reuter S., van Wijck Y., Urban S., Kyburz A., Maxeiner J., Martin H., Yogev N., Waisman A., Gerhard M., et al. Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proc. Natl. Acad. Sci. USA. 2014;111:11810–11815. doi: 10.1073/pnas.1410579111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Smoot D.T., Resau J.H., Earlington M.H., Simpson M., Cover T.L. Effects of Helicobacter pylori vacuolating cytotoxin on primary cultures of human gastric epithelial cells. Gut. 1996;39:795–799. doi: 10.1136/gut.39.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Harris P.R., Cover T.L., Crowe D.R., Orenstein J.M., Graham M.F., Blaser M.J., Smith P.D. Helicobacter pylori cytotoxin induces vacuolation of primary human mucosal epithelial cells. Infect. Immun. 1996;64:4867–4871. doi: 10.1128/iai.64.11.4867-4871.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fiocca R., Luinetti O., Villani L., Chiaravalli A.M., Capella C., Solcia E. Epithelial cytotoxicity, immune responses, and inflammatory components of Helicobacter pylori gastritis. Scand. J. Gastroenterol. Suppl. 1994;205:11–21. doi: 10.3109/00365529409091404. [DOI] [PubMed] [Google Scholar]
- 181.Terebiznik M.R., Vazquez C.L., Torbicki K., Banks D., Wang T., Hong W., Blanke S.R., Colombo M.I., Jones N.L. Helicobacter pylori VacA toxin promotes bacterial intracellular survival in gastric epithelial cells. Infect. Immun. 2006;74:6599–6614. doi: 10.1128/IAI.01085-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Amieva M.R., Salama N.R., Tompkins L.S., Falkow S. Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell. Microbiol. 2002;4:677–690. doi: 10.1046/j.1462-5822.2002.00222.x. [DOI] [PubMed] [Google Scholar]
- 183.Argent R.H., Thomas R.J., Letley D.P., Rittig M.G., Hardie K.R., Atherton J.C. Functional association between the Helicobacter pylori virulence factors VacA and CagA. J. Med. Microbiol. 2008;57:145–150. doi: 10.1099/jmm.0.47465-0. [DOI] [PubMed] [Google Scholar]
- 184.Yokoyama K., Higashi H., Ishikawa S., Fujii Y., Kondo S., Kato H., Azuma T., Wada A., Hirayama T., Aburatani H., et al. Functional antagonism between Helicobacter pylori CagA and vacuolating toxin VacA in control of the NFAT signaling pathway in gastric epithelial cells. Proc. Natl. Acad. Sci. USA. 2005;102:9661–9666. doi: 10.1073/pnas.0502529102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tegtmeyer N., Zabler D., Schmidt D., Hartig R., Brandt S., Backert S. Importance of EGF receptor, Her2/Neu and Erk1/2 kinase signalling for host cell elongation and scattering induced by the Helicobacter pylori CagA protein: Antagonistic effects of the vacuolating cytotoxin VacA. Cell. Microbiol. 2009;11:488–505. doi: 10.1111/j.1462-5822.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- 186.Akada J.K., Aoki H., Torigoe Y., Kitagawa T., Kurazono H., Hoshida H., Nishikawa J., Terai S., Matsuzaki M., Hirayama T., et al. Helicobacter pylori CagA inhibits endocytosis of cytotoxin VacA in host cells. Dis. Models Mech. 2010;3:605–617. doi: 10.1242/dmm.004879. [DOI] [PubMed] [Google Scholar]
- 187.Tsugawa H., Suzuki H., Saya H., Hatakeyama M., Hirayama T., Hirata K., Nagano O., Matsuzaki J., Hibi T. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–777. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]