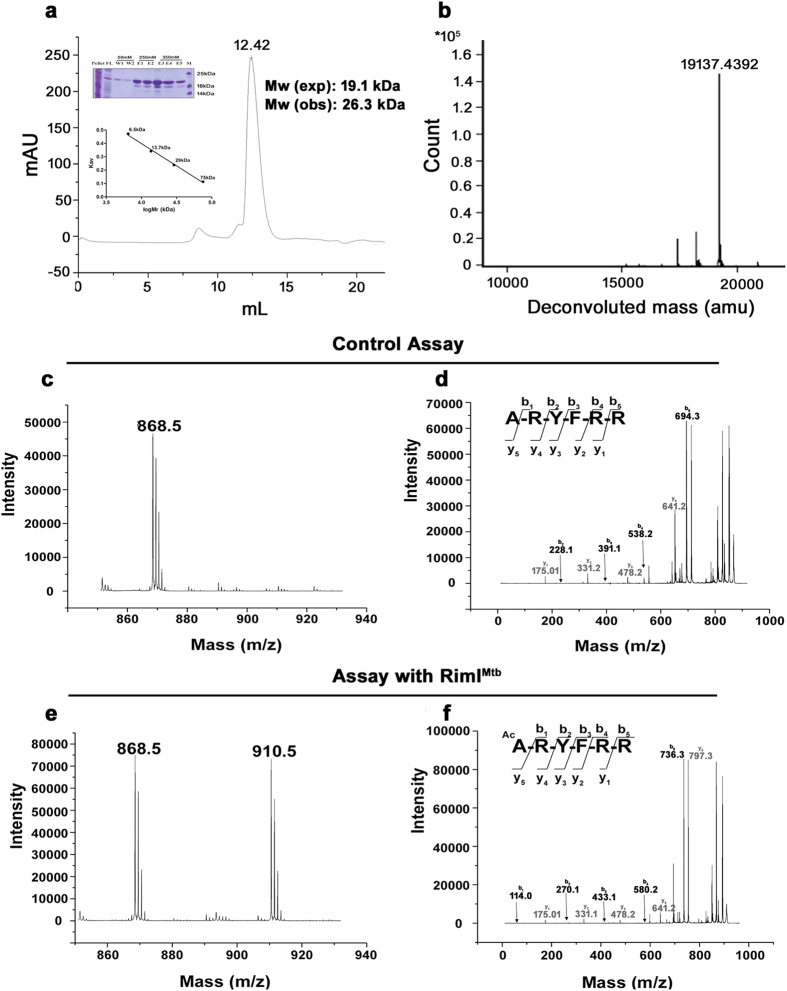
Figure 1. Purification of RimIMtb and identification of Nα-acetyltransferase activity of RimIMtb.
(a) Gel filtration of RimIMtb using superdex 75 10/300GL. Ni-NTA purified RimIMtb (as shown in protein gel) eluted as monomer (calibration curve given in inset) (b) Confirmation of intact mass (19.1 kDa) of purified RimIMtb monomer using LC-ESI-MS (c) MS analysis of control assay (without enzyme) using DPC peptide (ARYFRR) as substrate (d) MS/MS analysis of precursor ion (868.5 Da) of DPC peptide in control assay (e) MS analysis of enzyme assay confirming acetylation of DPC peptide by RimIMtb. Modified peptide was observed (910.5) with an increase of 42.0105 Da as compared to unmodified peptide (868.5 Da) concomitant to the addition of acetyl group (f) MS/MS analysis of modified (910.5 Da) precursor ion of the substrate (DPC) peptide. An increase of 42.0105 Da was observed in all the b-ions (in black) while the y-ions (in grey) remained the same as that of unmodified substrate, confirming the site of acetylation as the N-terminal amino acid i.e. Ala.