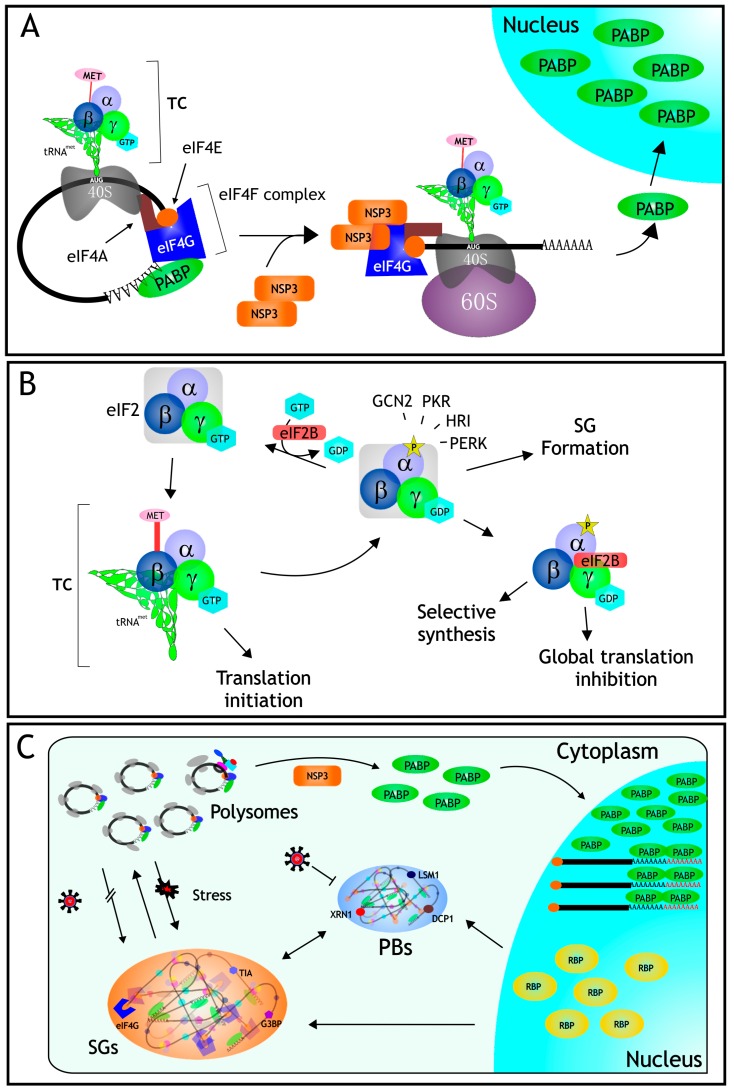
Figure 1.
Translation control in rotavirus infected cells. (A) During the infection, the nonstructural protein NSP3 interferes with the translation machinery by displacing PABP from its site of binding in eIF4G, and also by indirectly causing the nuclear relocalization of PABP, and the accumulation and hyperadenylation of poly(A)-containing mRNAs (see text); (B) eIF2 is an heterotrimer formed by subunits (α, β, and γ); it binds to GTP and tRNAMet forming the ternary complex (TC) which is in charge of loading the initial Met residue during protein translation. During rotavirus infection, the α subunit of eIF2 becomes phosphorylated by PKR, preventing the formation of the TC, and thus causing a global translation inhibition; (C) Even under these severe inhibitory translation conditions caused by the infection, the formation of stress granules (SGs) is prevented, and the amount of processing bodies (PBs) decreases, despite the fact that several of the RBPs that assemble these RNA granules exit from the nucleus (see text for details).