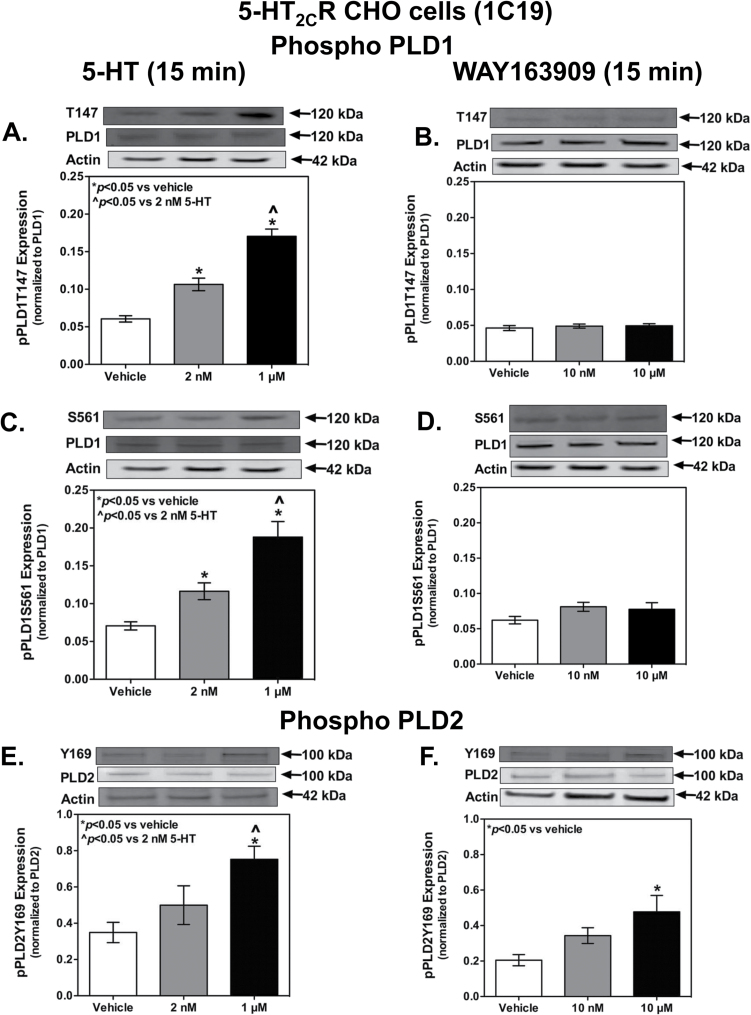
Figure 5.
Endogenous (5-hydroxytryptamine [5-HT]-) or selective agonist (WAY163909-) mediated activation (15 minutes) of stable but “low-expressing” (~250fmol/mg) unedited human isoform of the 5-hydroxytryptamine (5-HT) 2C receptor (5-HT2CR) in Chinese hamster ovary (CHO)-K1 cells increases phosphorylation states of the 2 different phospholipase D (PLD) isoforms in a dose-dependent manner. Whole cell homogenates were analyzed by Western blot (n=3–6 samples/each). Densitometric analyses were conducted on the (A-B) pPLD1T147 (120kDa), (C-D) pPLD1S561 (120kDa), (E-F) pPLD2Y169 (100kDa), (A-D) PLD1 (120kDa), and (E-F) PLD2 bands. Interestingly, 5-HT activation results in increased phosphorylation states for all 3 phosphorylation sites (A, C, E) tested, however, WAY163909 activation results in a significant increase only in the Y169 phosphorylated state at the highest concentration (F). Results represent the mean density (±SEM) of the respective IR band of each phosphorylated form normalized to the total protein levels. Actin was used as a loading control. *P<.05 vs vehicle; ^P<.05 vs 2nM 5-HT.