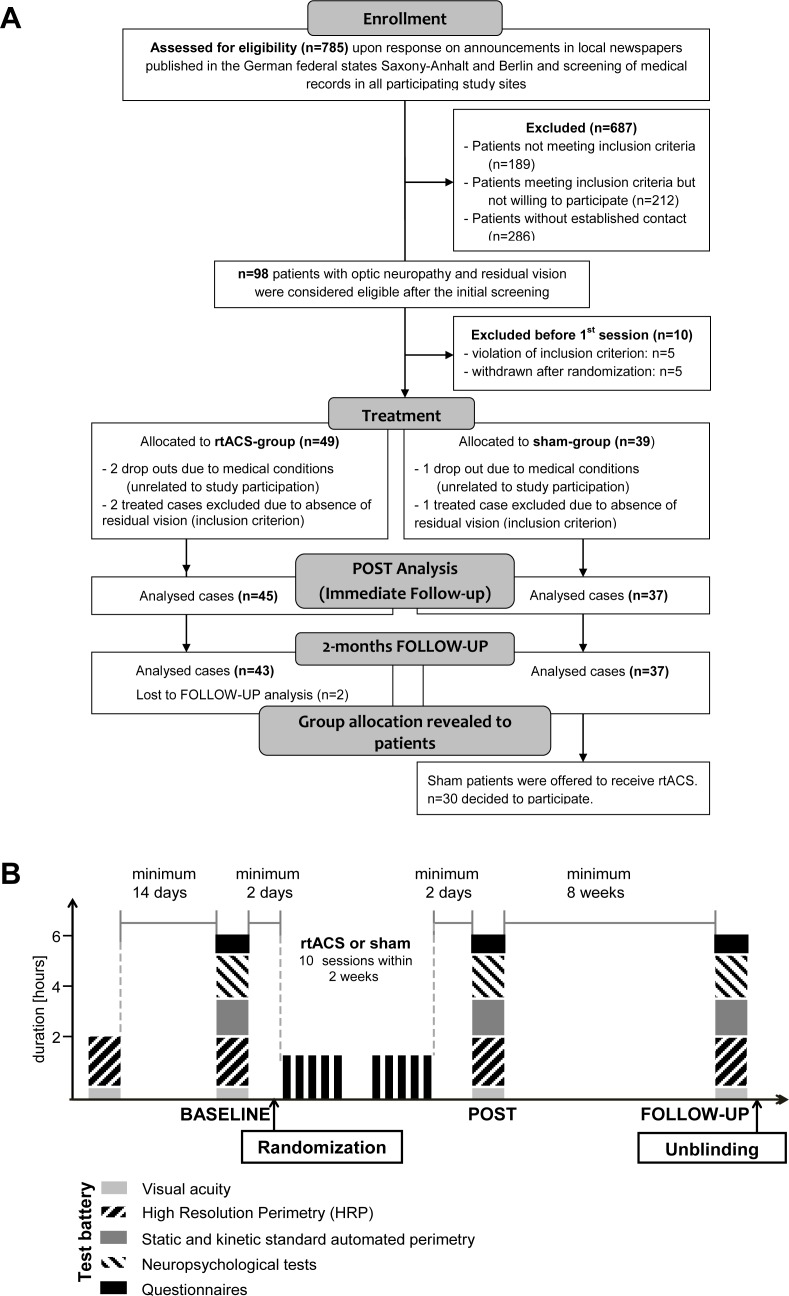
Fig 1. Consort flow chart and study design.
(A) Patient flow for cases included in the primary outcome measure analysis. Of 98 eligible patients, 45 were treated with rtACS and 37 with sham-stimulation. Five subjects left the study between initial screening and BASELINE for different reasons and another five subjects were excluded due to violation of an inclusion criterion (unacceptable fluctuations between initial screening and BASELINE). During the treatment phase three subjects dropped out because of medical conditions that were unrelated to study participation. Three treated cases of legally blind subjects were excluded from subsequent analyses due to violation of inclusion criterion (no residual vision). (B) Study design with diagnostic and treatment visits. Randomization was done after BASELINE assessment. Stability of VF defects was ascertained by comparing VFs at BASELINE with those obtained during the screening visit 2 weeks earlier. Upon completion of the 10-day treatment, all initial diagnostic tests were repeated (POST). The FOLLOW-UP diagnostic assessment was conducted after a therapy-free interval of at least 2 months.