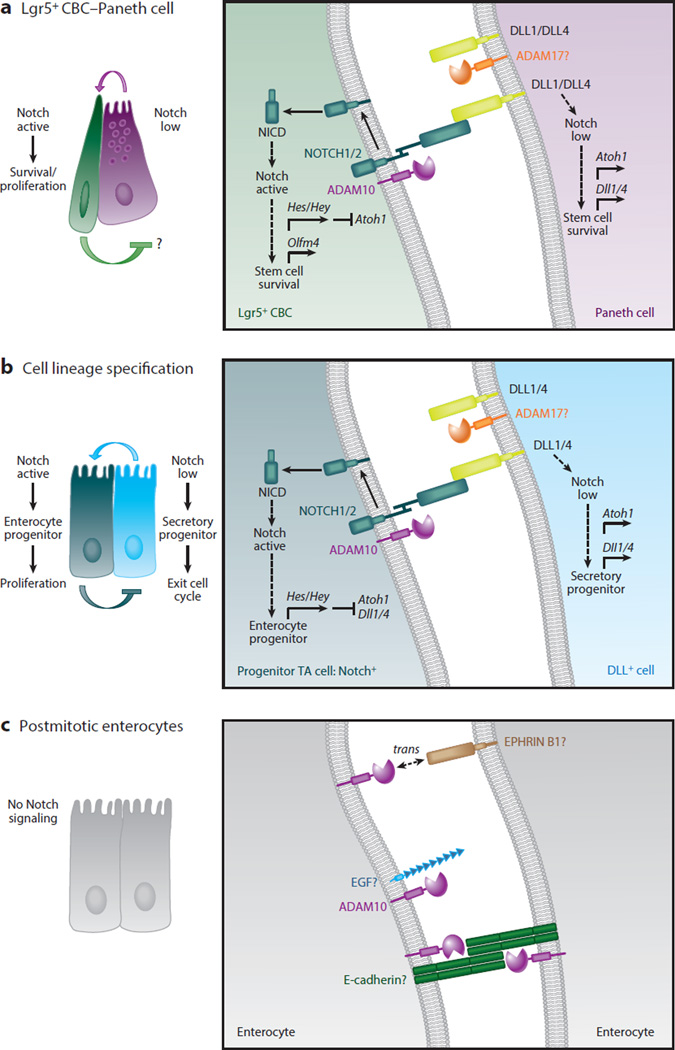
Figure 5.
Cell-autonomous A disintegrin and metalloproteinase (ADAM)10 signaling acts iteratively to regulate Notch signaling in intestinal stem cells (ISCs) and transit-amplifying (TA) progenitors during crypt homeostasis. (a) ADAM10-mediated Notch signaling is required for the survival and maintenance of Leu-rich repeat–containing G protein–coupled receptor 5–expressing (Lgr5+) crypt base columnar stem cells (CBCs). Lgr5+ CBCs expressing NOTCH1/2 receptors are intercalated between Paneth cells expressing DLL1 and DLL4 ligands in the small intestinal stem cell niche. High Notch activity in Lgr5+ CBCs is required for the proliferation and survival of stem cells and maintenance of the stem cell pool. Notch signaling is activated in Lgr5+ CBCs when Dll ligand, found on the surface of Paneth cells, binds to Notch receptors expressed on the surface of Lgr5+ CBCs. Several studies have shown that the intestine tolerates Paneth cell depletion, suggesting that Notch ligand is likely provided by other cells as well. Notch is sequentially cleaved by ADAM10 and γ-secretase to generate the Notch intracellular domain (NICD), which translocates to the nucleus, where its forms an active transcriptional complex. In Notch-active Lgr5+ CBCs, Notch targets genes including those that encode the Hes/Hey transcription factors, which repress Atoh1 and Dll1/4 ligand transcription and enhance expression of the stem cell marker Olfm4. In DLL+ Paneth cells, low Notch activity, reinforced through Notch lateral inhibition, allows derepression of Atoh1 and Dll1/4 ligand expression. ADAM10 is not required in Paneth cells to maintain the Lgr5+ CBC stem cell pool, but it may be involved in other redundant signaling pathways (e.g., EGF) that contribute to the stem cell niche. (b) ADAM10-mediated Notch signaling is required for the fate specification of TA cells. Classical Notch lateral inhibition determines whether a TA cell becomes an absorptive or secretory progenitor. In Notch-active TA progenitors, Notch targets genes including those that encode the Hes/Hey transcription factors, which repress Atoh1 and Dll1/4 ligand transcription. These cells are fated to become absorptive progenitors, which undergo several rounds of proliferation before differentiating into postmitotic enterocytes. In DLL+ TA cells, low Notch activity allows derepression of Atoh1 and Dll1/4 ligand expression. These cells are fated to become secretory progenitors, which rapidly exit the cell cycle and differentiate into distinct secretory cell types. Under certain experimental conditions, Notch ligands can be subject to extracellular cleavage by ADAM proteases such as ADAM17 (as shown in panels a and b). (c) ADAM10 signaling in postmitotic differentiated intestinal epithelial cells (IECs). Although Notch signaling is restricted to the crypt compartment, ADAM10 is abundantly expressed on all differentiated IECs. This implies that ADAM10 is involved in other shedding events in these postmitotic IECs. Potential ADAM10 substrates include E-cadherin, EGF, and EPHRIN B1. The profound effects of ADAM10-deficiency in the ISC/progenitor compartment have hindered analysis of other ADAM10 substrates in vivo.