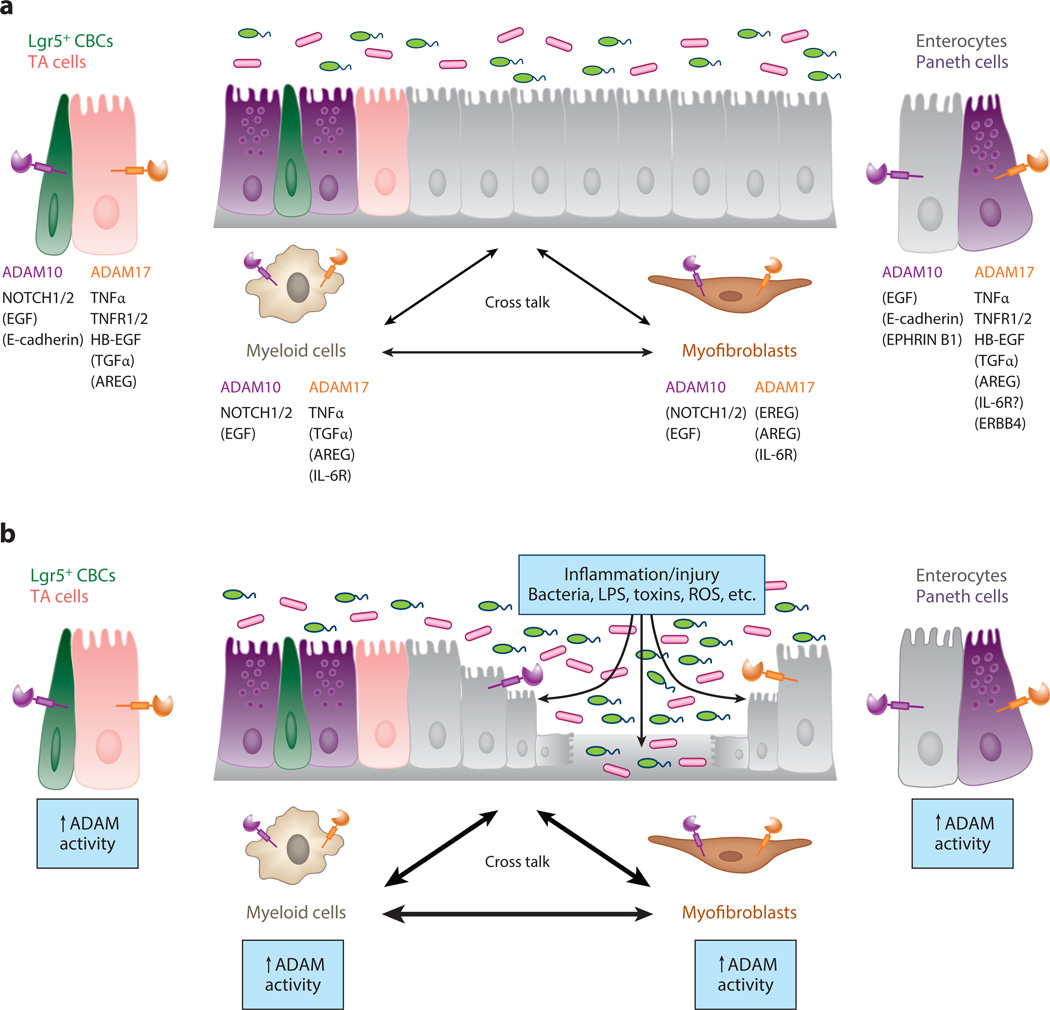
Figure 6.
A disintegrin and metalloproteinases (ADAMs) play a central role in intercellular communication during intestinal homeostasis and upon injury/inflammation. (a) A schematic diagram showing the specific roles of ADAM10 and ADAM17 signaling in cross talk between different cell types during normal intestinal homeostasis. For simplicity, only ADAM-mediated signaling between intestinal epithelial cells (IECs), myofibroblasts, and macrophages/myeloid cells is shown. ADAM activity is required in many other cell types of the gastrointestinal tract, including other immune cell populations (e.g., dendritic cells, T cells, and B cells), endothelial cells, and enteric neurons (not shown). For each cell type, ADAM substrate specificity and hierarchy may be different. For ADAM substrates, direct evidence exists for cleavage events in the gastrointestinal tract. For ADAM substrates listed in parentheses, in vitro data strongly suggest that ADAM-mediated cleavage events occur in the gastrointestinal tract. TA denotes transit amplifying. (b) Under conditions of intestinal inflammation and/or IEC injury, ADAM activity is upregulated. Loss of barrier integrity allows gut luminal contents to directly interact with ADAMs expressed on the basolateral surface of IECs. Numerous proinflammatory stimuli [e.g., lipopolysaccharide (LPS), reactive oxygen species (ROS), cytokines] will stimulate ADAM expression and proteolytic activity. Normally, enhanced ADAM activity and signaling cross talk are carefully integrated to resolve inflammation/injury and promote epithelial restitution and regeneration. However, under conditions of chronic and relapsing inflammation, these same signals, if sustained, may increase the risk of inflammation-associated cancer.