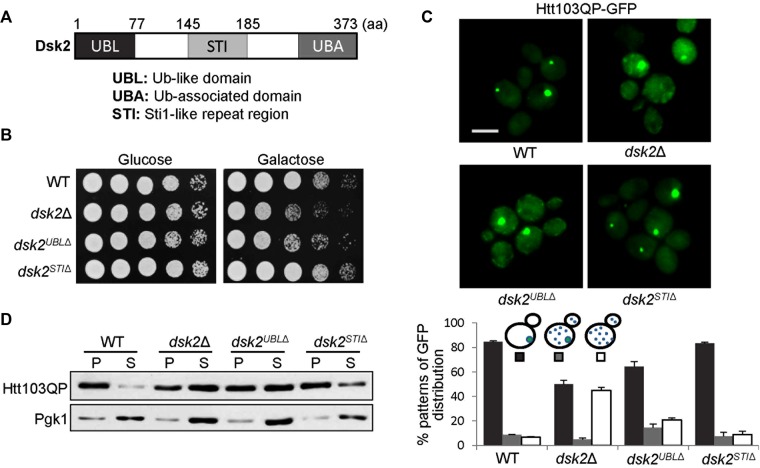
FIGURE 2:
Deletion of the DSK2 UBL domain causes defective Htt103QP IB formation. (A) Schematic illustration of the domains of the yeast Dsk2 protein. (B) dsk2UBL∆ and dsk2∆ mutants show sick growth when Htt103QP is overexpressed. Cells were grown to saturation, 10-fold diluted, and spotted onto glucose and galactose plates for 2 d of incubation at 30°C. (C) dsk2UBL∆ mutants show defective Htt103QP IB formation similar to dsk2∆ mutants. WT and dsk2∆, dsk2UBLΔ, and dskSTIΔ mutant cells with Htt103QP plasmid were grown in galactose medium for 12 h at 30°C to induce Htt103QP expression. Top, GFP signal in some representative cells by fluorescence microscopy. Bottom, percentage of cells with different patterns of GFP distribution (n > 100). The result is the average of three separate experiments. Scale bar, 5 μm. (D) dsk2UBLΔ and dsk2Δ mutants show more detergent-soluble Htt103QP. Cells with the indicated genotypes were grown in galactose medium for 12 h. Cell lysates were prepared and divided into detergent-soluble (supernatant) and -insoluble (pellet) fractions after centrifugation. Htt103QP protein levels were determined by Western blotting with anti-GFP antibody. Pgk1 levels were used as a loading control.