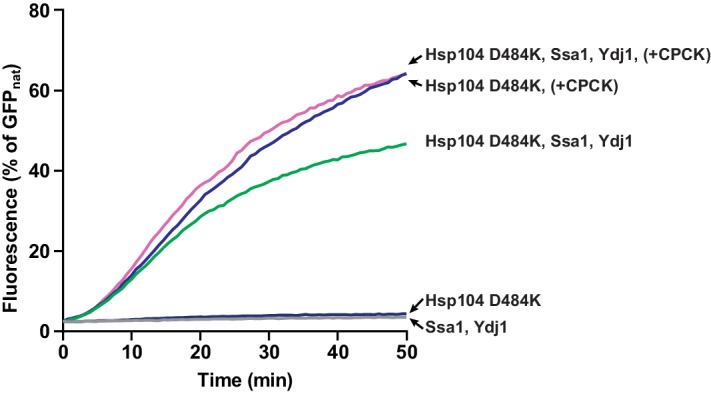
Figure 3. The derepressed D484K variant of Hsp104 is inhibited by ADP.
(A) The ATPase activity of Hsp104 D484K is strongly affected by ADP. ATPase activity of D484K variant was measured at 10 mM ATP and at the indicated concentrations of ADP. Values are the average of three independent experiments (± SD). (B) ADP inhibits the disaggregation activity of Hsp104 D484K in the absence of Hsp70. Disaggregation of heat-aggregated GFP (0.04 mg ml-1) by Hsp104 D484K (0.5 μM) at 10 mM ATP (blue). After 60 s of the reaction, ADP was added to 2 mM concentration (red). (C) ATP regeneration system or (D) The Hsp70 system restores the disaggregation activity of Hsp104 D484K. The experiment was initiated as in (B), and after 5 min (C) an ATP regeneration system comprising PK (0.1 mg ml-1) and PEP (40 mM) (purple) or (D) the Hsp70 chaperone system: Ssa1 (2 μM) and Ydj1 (0.5 μM) (green) was added to the reaction mixture.
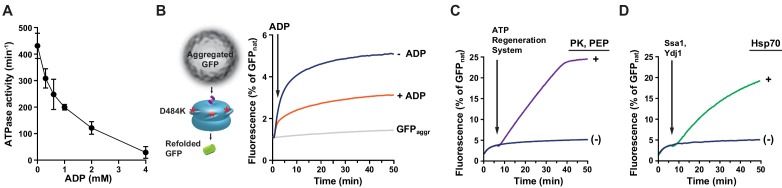
Figure 3—figure supplement 1. Hsp104 D484K is affected by ADP similarly as the WT Hsp104.
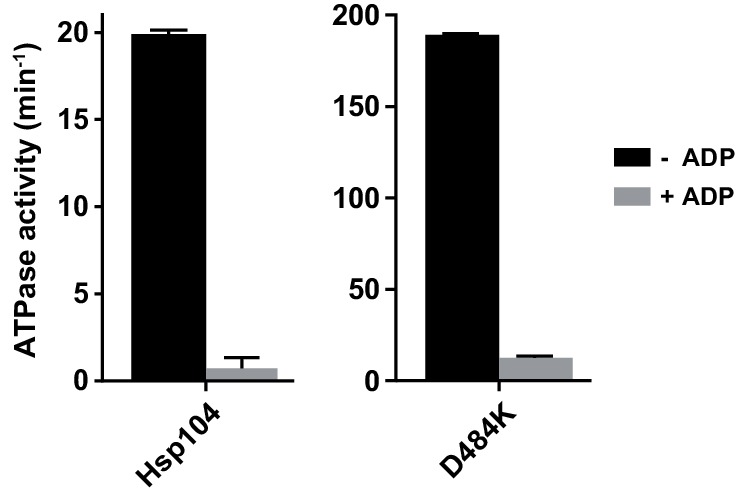
Figure 3—figure supplement 2. Protein translocation activity of HAP D484K is inhibited by ADP.
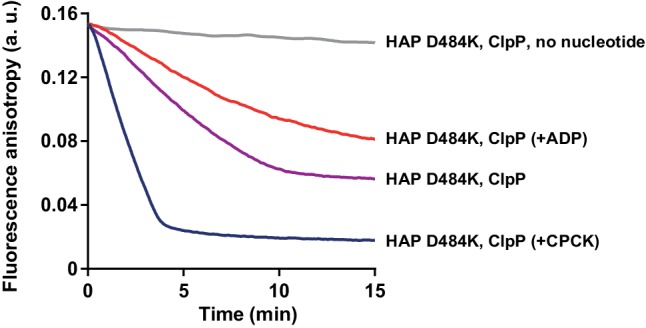
Figure 3—figure supplement 3. Hsp70 allows efficient disaggregation at low ATP:ADP ratio.
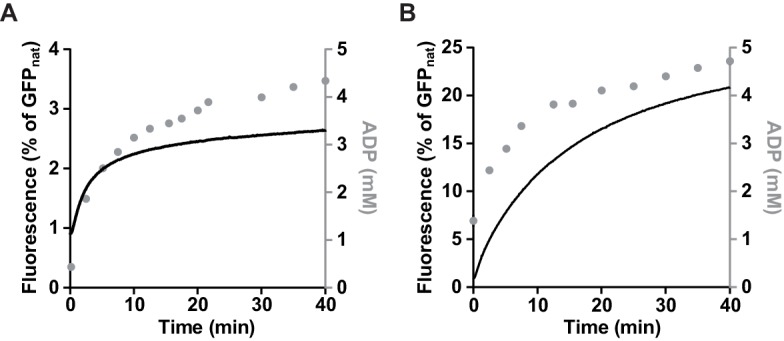
Figure 3—figure supplement 4. In the absence of ADP the derepressed Hsp104 D484K is independent of Hsp70 in disaggregation.