Figure 5. Hsp70 does not support Hsp104 in processing of disordered, non-aggregated proteins.
Proteolysis of fRCMLa (5 μM) by HAP (1 μM) and ClpP (1.8 μM), carried out at 2.6 mM ATP with or without 1 mM ADP and in the presence or absence of Ssa1 (2 μM) and Ydj1 (0.5 μM), as indicated. Grey line shows a control experiment, in which HAP was omitted. a. u. – arbitrary units.
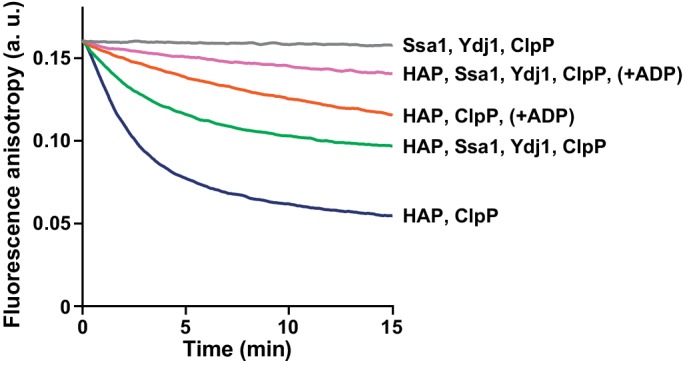
Figure 5—figure supplement 1. Hsp70 interaction with fRCMLa.
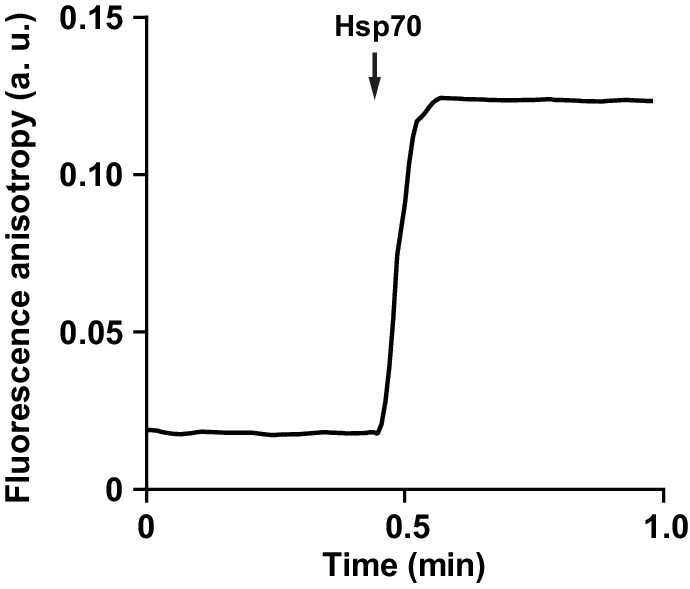
Figure 5—figure supplement 2. Hsp70 does not support Hsp104 in processing of α-casein.
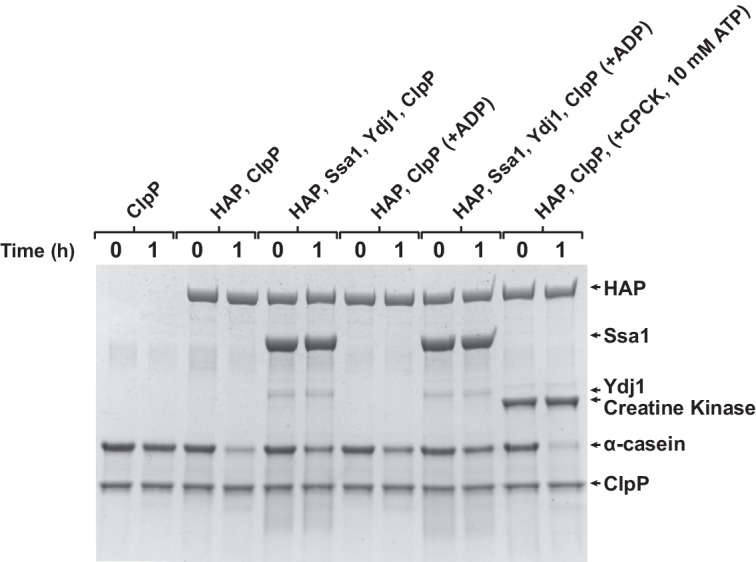
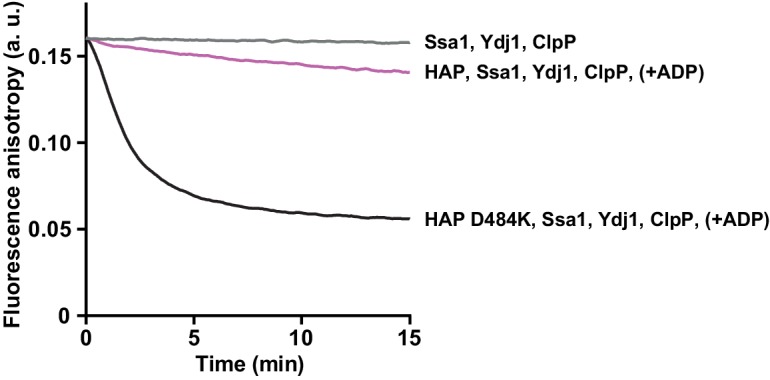
Figure 5—figure supplement 3. Derepressed HAP D484K is efficient in translocation of disordered proteins at the physiological ATP and ADP concentrations.