Table 2.
NMR Data for 3-O-propyl-2,3-dehydrosilibinin (5) (1H NMR: 300 MHz; 13C NMR: 75 MHz).
| Position | 3-O-propyl-2,3-dehydrosilibinin (acetone-d6) | |
|---|---|---|
| δC, type | δH, (J in Hz) | |
|
|
|
|
| 2 | 147.1, C | - |
| 3 | 138.8, C | - |
| 4 | 175.4, C | - |
| 4a | 105.9, C | - |
| 5 | 163.2, C | - |
| 6 | 99.4, CH | 6.25, s |
| 7 | 165.0, C | - |
| 8 | 94.5, CH | 6.52, s |
| 8a | 157.8, C | - |
| 10 | 80.0, CH | 4.25–4.23, m |
| 11 | 77.2, CH | 5.05, d (7.8) |
| 12a | 144.8, C | - |
| 13 | 118.0, CH | 7.70, s |
| 14 | 124.4, C | - |
| 15 | 123.2, CH | 7.74, d (8.1) |
| 16 | 117.7, CH | 7.06, d (8.1) |
| 16a | 156.2, C | - |
| 17 | 128.9, C | - |
| 18 | 111.9, CH | 7.16, s |
| 19 | 148.6, C | - |
| 20 | 148.1, C | - |
| 21 | 115.8, CH | 6.90, d (8.1) |
| 22 | 121.7, CH | 7.00, d (8.1) |
| 23 | 61.7, CH2 | 3.80, br.d (12.3) |
| 3.55, br.d (12.3) | ||
|
| ||
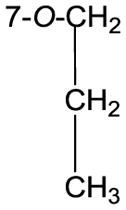
|
74.8, CH2 | 4.02, t (6.6) |
| 24.0, CH2 | 1.73, Hex (7.2) | |
| 10.8, CH3 | 0.96, t (7.5) | |
|
| ||
| 19-OMe | 56.3, CH3 | 3.95, s |
| 5-OH | - | 11.67, s |
| 20-OH | - | 5.79, s |
| 23-OH | - | |
