Abstract
We evaluated the effect of cognitive stimulation (CS) on platelet total phospholipases A2 activity (tPLA2A) in patients with mild cognitive impairment (MCI_P). At baseline, tPLA2A negatively correlated with Mini-Mental State Examination score (MMSE_s): patients with MMSE_s <26 (Subgroup 1) had significantly higher activity than those with MMSE_s ≥26 (Subgroup 2), who had values similar to the healthy elderly. Regarding CS effect, Subgroup 1 had a significant tPLA2A reduction, whereas Subgroup 2 did not significantly changes after training. Our results showed for the first time that tPLA2A correlates with the cognitive conditions of MCI_P, and that CS acts selectively on subjects with a dysregulated tPLA2A.
Keywords: Blood platelets, cognitive stimulation, mild cognitive impairment, phospholipases A2
INTRODUCTION
Phospholipases A2 (PLA2) form a superfamily of enzymes that catalyze production of lyso-phospholipids and free fatty acids by the hydrolysis of phospholipids sn-2 ester bond. They play a pivotal role in many physiological processes, including membrane remodelingand cell signaling [1, 2], and are involved in neurodegenerative disorders [3, 4].
PLA2 modulation is a potential therapeutic target [5, 6]; in this context, cognitive stimulation (CS) is particularly promising, not only because in animal models it has effective regulating properties [7], but also because it is non-invasive, has no side effects, and presents no contraindications.
To date, only one study has been performed in humans: in a little cohort of healthy elderly subjects, a memory training intervention was proved to modulate platelet PLA2 activity [8]. The use of platelet PLA2 as peripheral biomarker of the neuronal enzyme is convincing in light of the recent finding that total PLA2 (tPLA2) activity in thrombocytes may mirror the total activity in the brain [9]. Moreover, platelets are widely considered “circulating neurons” because of the similarities existing between the two cells in terms of enzymes, receptors, and metabolic products [10, 11].
On these grounds, we evaluated the effects of CS on platelet tPLA2 activity in a cohort of subjects with mild cognitive impairment (MCI).
MATERIALS AND METHODS
Subjects
All the subjects (74 healthy and 70 MCI) were enrolled at the Geriatrics Operative Unit of INRCA (Italian National Research Centres on Aging) in Fermo (Italy). The research was approved by the Institutional Ethical Committee (code SC/12/301) and each participant provided informed consent to participate to the study.
All subjects underwent a complete medical, neuropsychological, and functional evaluation; moreover, several laboratorial parameters (such as thyroid hormones, vitamin B12, and folic acid) as well as neuroimaging analyses (PET, CT, or MRI) were assessed to exclude any alterations that can determine cognitive deficits. MCI was diagnosed according to the criteria of Petersen et al. [12]. Patients under benzodiazepines, antidepressants, lipid lowering medications, non-steroidal anti-inflammatory drugs, anticoagulants, antihypertensive, and corticosteroids were included, and possible influence on platelet tPLA2 activity was specifically evaluated.
The main characteristics of the populations are summarized in Supplementary Table 1A; inclusion and exclusion criteria are as in Casoli et al. [13].
Cognitive training
Each MCI subject was randomly assigned to either a multi-component cognitive training exercise group (EG; n = 37) or a control group (CG; n = 33) whose members received only some suggestions to improve specific outcomes. The protocol of cognitive training was applied as described [13]. Performances in digits span forward (auditory verbal short term memory [14]), Corsi supraspan (visuospatial short term memory [14]), attentive matrices (selective attention [15]), phonemic verbal and semantic fluency (linguistic abilities [16]), immediate and delayed prose recall (prose memory [15]), as well as word pairs learning (learning [16]) tests were used as outcomes.
Platelets isolation
Thirty milliliters of lithium heparin whole blood were drawn from each subject before cognitive training (baseline) and after termination (follow up (FU)). All drawings were done between 8:00 and 9:00 AM, in fasting state. Platelets were separated according to Rosenberg et al. [17], sonicated on ice (30 s at 8μm of amplitude) and centrifuged at 10,000×g for 15 min at 4°C. Supernatant aliquots were immediately stored at – 80°C and tested within a month. Protein concentration was determined by the Lowry method [18]. Where not differently specified, all procedures were performed at room temperature.
tPLA2 activity determination
Enzymatic activity was determined by a commercial kit (cPLA2 Assay Kit, Cayman Chemical Company, Michigan, USA), normalized by protein concentration and expressed as nmol/min/mg. Since samples were not preliminarily purified for sPLA2 or treated by iPLA2-specific inhibitors, the data obtained can be referred to tPLA2. All samples were measured in duplicates.
Statistical analyses
Results were expressed as means ± standard error of the mean (SEM) (continuous variables) or as percentage (categorical variables). Statistical comparisons were performed by t-Student test or by χ2 test to compare the two groups at baseline; by paired t-Student test to evaluate the differences before and after the CS period; and by Pearson’s coefficient to assess correlation between variables. The significance was accepted for p < 0.05.
RESULTS
The compliance to the study was 93.5% in CG and 87.9% in EG. “Not Evaluated” subjects at FU included those who did not complete the cognitive training/were not cognitively retested (dropout) or did not allow the drawn at the FU step; the overall dropout rate was 2.9% . The analyses performed at baseline included all the individuals enrolled.
Cognitive outcomes
Table 1 summarizes the cognitive outcomes of MCI patients evaluated at baseline and FU. In thesepreliminary results, EG evidenced significantly increased performances at FU in attentive matrices, phonemic verbal fluency as well as immediate, delayed, and total prose recall tests. No significant differences were envisaged comparing baseline and FU in CG.
Table 1.
Cognitive outcomes of control (CG) and trained (EG) MCI patients analyzed at baseline and FU
| Baseline score | FU score | p | ||
| Digits forward Test | CG | 4.56 ± 0.130 | 4.47 ± 0.136 | 0.500 |
| EG | 4.36 ± 0.151 | 4.65 ± 0.169 | 0.103 | |
| Corsi supraspan Test | CG | 5.12 ± 0.125 | 5.01 ± 0.134 | 0.476 |
| EG | 4.84 ± 0.165 | 5.17 ± 0.156 | 0.075 | |
| Attentive Matrices Test | CG | 41.32 ± 1.574 | 40.04 ± 1.433 | 0.146 |
| EG | 39.65 ± 1.661 | 43.39 ± 1.603 | 0.010 | |
| Semantic verbal fluency Test | CG | 2.42 ± 0.240 | 2.40 ± 0.229 | 0.702 |
| EG | 1.84 ± 0.239 | 2.13 ± 0.283 | 0.182 | |
| Phonemic Verbal fluency Test | CG | 24.83 ± 1.365 | 24.01 ± 1.033 | 0.404 |
| EG | 28.46 ± 1.315 | 31.27 ± 1.601 | 0.018 | |
| Immediate prose recall Test | CG | 3.25 ± 0.398 | 3.09 ± 0.398 | 0.755 |
| EG | 3.26 ± 0.383 | 4.23 ± 0.354 | 0.037 | |
| Delayed prose recall Test | CG | 3.55 ± 0.499 | 2.81 ± 0.453 | 0.119 |
| EG | 2.98 ± 0.448 | 4.11 ± 0.443 | 0.038 | |
| Total prose recall Test | CG | 7.55 ± 0.761 | 6.71 ± 0.668 | 0.241 |
| EG | 6.83 ± 0.683 | 8.80 ± 0.666 | 0.006 | |
| Word pairing Test | CG | 6.91 ± 0.536 | 7.04 ± 0.501 | 0.802 |
| EG | 8.65 ± 0.723 | 8.98 ± 0.824 | 0.517 |
In none of the tests, CG and EG showed statistical differences at baseline. The significant p values are marked in bold.
Platelet tPLA2 activity at baseline
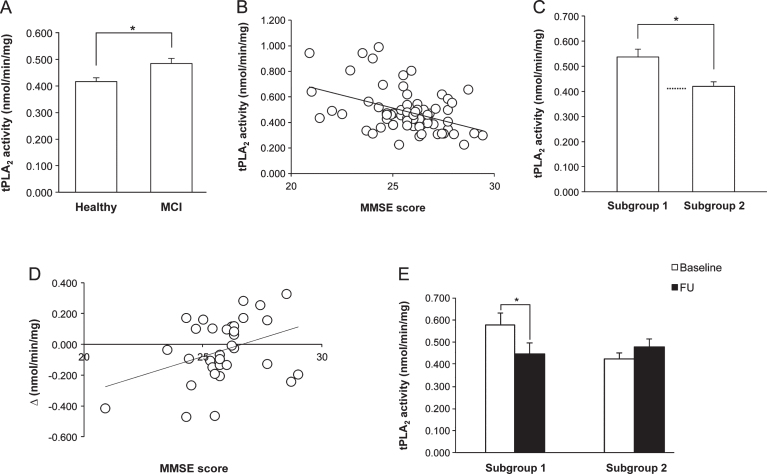
At baseline, tPLA2 activity of MCI patients was significantly higher (p = 0.008) than that of healthy subjects (Fig. 1A), and the significance was maintained also when the data were adjusted for age and schooling by multiple linear regression analysis.
Fig.1.
A). Platelet tPLA2 activity at baseline in healthy elderly and MCI subjects, who showed a significantly higher value. B) Correlation between MMSE score and tPLA2 activity in MCI patients at baseline. Note that when MMSE values are higher, enzymatic activity is lower. C) tPLA2 activity of MCI patients at baseline falls into two groups on the bases of the MMSE score: subjects with MMSE score <26 (Subgroup 1) had enzymatic activity significantly higher than subjects with MMSE score ≥26 (Subgroup 2), whose values were comparable to those of healthy elderly (dotted line). D) Correlation between the MMSE score at baseline and tPLA2 activity changes before and after the intervention (Δ), in the experimental group of MCI patients. Δ= tPLA2 activity at FU- tPLA2 activity at baseline. E) Effect of cognitive stimulation on tPLA2 activity of MCI patients Subgroups 1 and 2. Only in Subgroup 1, the training induced a significant decrease.
In the MCI group, a significant negative correlation was envisaged between the Mini-Mental State Examination (MMSE) score and the tPLA2 activity (R = – 0.425, p < 0.001) (Fig. 1B). The significance did remain also when the cohort was stratified for potentially confounding variables (i.e., gender, marital status, schooling, and age of pathology onset). To further analyze the correlation, the MCI group was divided according to the MMSE value, using the median as cut-off point: subjects with a score <26 (Subgroup 1, n = 38) had significantly higher tPLA2 activity (p = 0.003) than individuals with a score ≥26 (Subgroup 2, n = 32), who showed values similar to the healthy elderly (Fig. 1C). The main characteristics of the two Subgroups are summarized in Supplementary Table 1B.
Effect of CS on platelet tPLA2 activity
No significant differences were found between enzymatic activity at baseline and FU in controls (0.479 ± 0.0293 versus 0.499 ± 0.0445) or in experimental individuals (0.502 ± 0.0341 versus 0.476 ± 0.0277). However, in EG, a significant positive correlation was observed between tPLA2 activity changes before and after the intervention (Δ is positive when the activity increases and negative when it decreases) and the MMSE score at baseline (R = 0.366, p = 0.049) (Fig. 1D); no significant correlation was found in controls (R = – 0.078, p = 0.675), indicating that this phenomenon is training-specific. Thus, analyzing the CS effect in the two subgroups identified on the bases of the MMSE score at baseline, tPLA2 activity showed a significant decrease in Subgroup 1 (p = 0.019), and no significant differences in Subgroup 2 at FU (Fig. 1E).
Drug influence
Drug use did not influence tPLA2 activity, with the exception of antidepressants in the MCI group: patients (n = 11) who used these drugs had significantly lower values at baseline in comparison to untreated MCI subjects (0.417 ± 0.0255 versus 0.496 ± 0.0231, p = 0.028). Excluding these 11 subjects, the significant differences and correlations remained unchanged.
DISCUSSION
The present study showed that in subjects with MCI, platelet tPLA2 activity correlates with patients’ cognitive conditions, and that CS acts selectively on the enzyme, i.e., it modulates the parameter only in individuals with deregulated values in comparison to the healthy elderly.
Based on the MMSE score, it was possible to subdivide at baseline the MCI cohort into two subgroups: patients with more evident cognitive impairment (MMSE score <26) and significantly higher tPLA2 activity, and individuals cognitively more preserved (MMSE score ≥26), who had tPLA2 activity similar to the healthy elderly. The finding that the increase of tPLA2 activity and the severity of the global cognitive status impairment are significantly linked suggests a possible role of tPLA2 in MCI progression. PLA2 activity alterations may lead to the synthesis of excessive proinflammatory mediators and peroxidative products [19], and inflammation and oxidative stress may contribute to the pathogenesis of Alzheimer’s disease (AD) [20, 21], of which MCI could be a prodromal condition. It is therefore conceivable that the more deregulated tPLA2 is, the more harmful molecules might be released, and the more severe the pathological consequences might become. The finding that in patients affected by AD tPLA2 activity is significantly higher than in healthy controls [22, 23] as well as in MCI subjects [23] is in line with this hypothesis.
As far as the therapeutic potentialities of CS are concerned, the protocol not only exerted positive effects on several cognitive outcomes, but also counteracted the peripheral enzymatic deregulation. Indeed, CS improved parameters linked to memory, attention, and verbal, confirming the results of others [24]. It is worth noting that CS acts on tPLA2 activity in a “dysfunction-dependent” mode: in subjects with an initial enzymatic activity higher than in the healthy elderly (Subgroup 1), CS reduced the value; in subjects with an initial enzymatic activity similar to the healthy elderly (Subgroup 2) CS did not induce any significant change. Thus, CS seems to have homeostatic properties on tPLA2 activity. This result may seem in contradictionwith the observation that in the healthy elderly CS induces platelet tPLA2 increase [8]. Actually, it is conceivable that, in absence of pathology, increased activity produced by the training improves cell functioning while in MCI, where the increased values might be linked to inflammation and oxidative stress, the protocol acts in the opposite way. Indeed, recent evidence supports the use of specific PLA2 inhibitors as preventive/therapeutic agents for inflammatory disorders [25], and several studies showed that environmental enrichment exert anti-inflammatory and neuromodulatory effects [26]. Thus, in MCI and AD, where the involvement of neuroinflammation is well established [27, 28], CS may produce a down regulation effect in the central nervous system, which might influence also circulation blood components.
In conclusion, this study suggests that platelet tPLA2 activity may be useful as peripheral biomarker to differentiate MCI patients at different pathological stages, and sustains the use of CS as non-pharmacological therapeutic strategy.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank all participants to the study and their families for the time they spent and for their willingness to collaborate. The authors also thank Mrs. Belinda Giorgetti and Mr. Moreno Solazzi for their technical assistance. The study was supported by the “Ricerca Finalizzata” grant 154/GR-2009-1584108 funded by the Italian Ministry of Health and the Marche Region.
Authors’ disclosures available online (http://www.j-alz.com/manuscript-disclosures/15-0714r1).
Appendix
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-150714.
REFERENCES
- [1]. Brown WJ, Chambers K, Doody A (2003) Phospholipase A2 (PLA2) enzymes in membrane trafficking: Mediators of membrane shape and function. Traffic 4, 214–221. [DOI] [PubMed] [Google Scholar]
- [2]. Murakami M, Kudo I (2002) Phospholipase A2. J Biochem 131, 285–292. [DOI] [PubMed] [Google Scholar]
- [3]. Sun GY, Shelat PB, Jensen MB, He Y, Sun AY, Simonyi A (2010) Phospholipase A2 and inflammatory responses in the central nervous system. Neuromolecular Med 12, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Gentile MT, Reccia MG, Sorrentino PP, Vitale E, Sorrentino G, Puca AA, Colucci-D’Amato L (2012) Role of cytosolic calcium-dependent phospholipase A2 in Alzheimer’s disease pathogenesis. Mol Neurobiol 45, 596–604. [DOI] [PubMed] [Google Scholar]
- [5]. Burke JE, Dennis EA (2009) Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res 50 Suppl S237–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Ong WY, Farooqui T, Kokotos G, Farooqui AA (2015) Synthetic and netural inhibitors of phospholipases A2: Their importance for understanding and treatment of neurological disorders. ACS Chem Neurosci 17, 814–831. [DOI] [PubMed] [Google Scholar]
- [7]. Schaffer EL, Forlenza OV, Gattaz WF (2009) Phospholipase A2 activation as a therapeutic approach for cognitive enhancement in early-stage Alzheimer disease. Psychopharmacology (Berl) 202, 37–51. [DOI] [PubMed] [Google Scholar]
- [8]. Talib LL, Valente KD, Vincentiis S, Gattaz WF (2013) Correlation between platelet and brain PLA(2) activity. Prostaglandins Leukot Essent Fatty Acids 89, 265–268. [DOI] [PubMed] [Google Scholar]
- [9]. Talib LL, Yassuda MS, Diniz BS, Forleanza OV, Gattaz WF (2008) Cognitive training increases platelet PLA2 activity in healthy elderly subjects. Prostaglandins Leukot Essent Fatty Acids 78, 265–269. [DOI] [PubMed] [Google Scholar]
- [10]. Barradas MA, Mikhailidis DP (1993) The use of platelets as models of neurons: Possible applications to the investigation of eating disorders. Biomed Pharmacother 47, 11–18. [DOI] [PubMed] [Google Scholar]
- [11]. Casoli T, Balietti M, Giorgetti B, Solazzi M, Scarpino O, Fattoretti P (2013) Platelets in Alzheimer’s disease-associated cellular senescence and inflammation. Curr Pharm Des 19, 1727–1738. [PubMed] [Google Scholar]
- [12]. Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST (2001) Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 56, 1133–1142. [DOI] [PubMed] [Google Scholar]
- [13]. Casoli T, Giuli C, Balietti M, Giorgetti B, Solazzi M, Fattoretti P (2014) Effect of cognitive training on the expression of brain-derived neurotrophic fcator in lymphocytes of mild cognitive impairment patients. Rejuvenation Res 17, 235–238. [DOI] [PubMed] [Google Scholar]
- [14]. Orsini A, Grossi D, Capitani E, Laiacona M, Papagno C, Vallar G (1987) Verbal and spatial immediate memory span: Normative data from 1355 adults and 1112 children. Ital J Neurol Sci 8, 539–548. [DOI] [PubMed] [Google Scholar]
- [15]. Spinnler H, Tognoni G (1987) Standardizzazione e taratura italiana di test neuropsicologici. Ital J Neurol Sci 6, 1–120. [PubMed] [Google Scholar]
- [16]. Novelli G, Papagno C, Capitani E, Laiacona M, Cappa SF, Vallar G (1986) Tre test clinici di memoria a lungo termine. Arch Psicol Neurol Psichiatr 47, 278–296. [Google Scholar]
- [17]. Rosenberg RN, Baskin F, Fosmire JA, Risser R, Adams P, Svetlik D, Honig LS, Cullum CM, Weiner MF (1997) Altered amyloid protein processing in platelets of patients with Alzheimer disease. Arch Neurol 54, 139–144. [DOI] [PubMed] [Google Scholar]
- [18]. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin Phenol reagent. J Biol Chem 193, 265–275. [PubMed] [Google Scholar]
- [19]. Farooqui AA, Harrocks LA (2006) Phospholipase A2-generated lipid mediators in the brain: The good, the bad, and the ugly. Neuroscientist 12, 245–260 . [DOI] [PubMed] [Google Scholar]
- [20]. Takeda S, Sato N, Morishita R (2014) Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: Relevance to pathogenesis and therapy. Front Aging Neurosci 6, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Sultana R, Butterfield DA (2010) Role of oxidative stress in the progression of Alzheimer’s disease. J Alzheimers Dis 19, 341–353. [DOI] [PubMed] [Google Scholar]
- [22]. Krzystanek E, Krzystanek M, Opala G, Trzeciak HI, Siuda J, Malecki A (2007) Platelet phospholipase A2 activity in patients with Alzheimer’s disease, vascular dementia and ischemic stroke. J Neural Transm 114, 1033–1039. [DOI] [PubMed] [Google Scholar]
- [23]. Gattaz WF, Talib LL, Schaeffer EL, Diniz BS, Forlenza OV (2014) Low platelet iPLA activity predicts conversion from mild cognitive impairment to Alzheimer’s disease: A 4-year follow-up study. J Neural Transm 121, 193–200. [DOI] [PubMed] [Google Scholar]
- [24]. Rodakowski J, Saghafi E, Butters MA, Skidmore ER (2015) Non-pharmacological interventions for adults with mild cognitive impairment and early stage dementia: An updated scoping review. Mol Aspects Med 43-44, 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Yarla NS, Bishayee A, Vadlakonda L, Chintala R, Duddukuri GR, Reddanna P, Kaladhar DS (2015) Phospholipase A2 isoforms as novel targets for prevention and treatment of inflammatory and oncologic diseases. Curr Drug Targets, doi: 10.2174-1389450116666150727122501. [DOI] [PubMed] [Google Scholar]
- [26]. Singhal G, Jaehne EJ, Corrigan F, Baune BT (2014) Cellular and molecular mechanisms of immunomodulation in the brain through environmental enrichment. Front Cell Neurosci 8, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Steardo L Jr, Brozuoli MR, Iacomino A, Esposito G, Steardo L, Scuderi C (2015) Does neuroinflammation turn on the flame in Alzheimer’s disease? Focus on astrocytes. Front Neurosci 9, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Heppner FL, Ransohoff RM, Cecher B (2015) Immune attack: The role of inflammation in Alzheimer disease. Nat Rev Neurosci 16, 358–372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-150714.