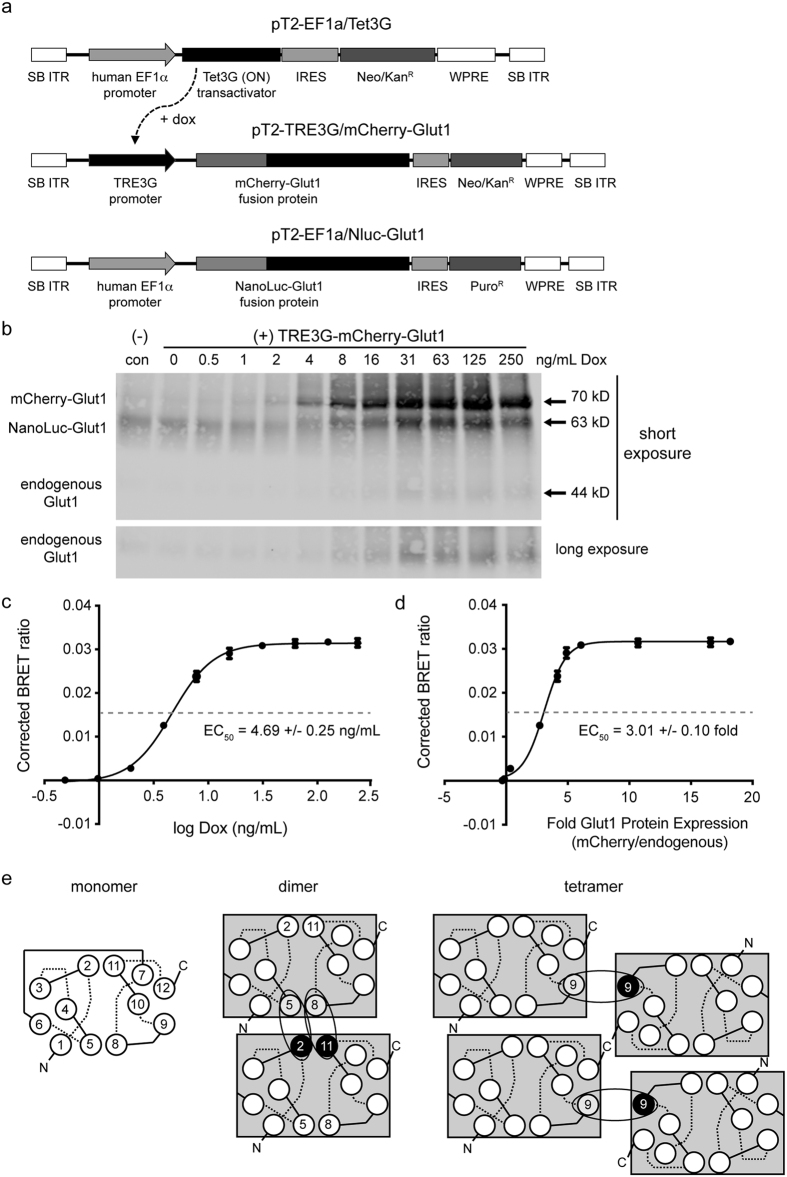
Figure 6. Determination of the relative GLUT1 density required for the formation of higher order membrane complexes.
(a) Maps of the Sleeping Beauty transposable element vectors used for stable integration of Nluc-GLUT1 and a tetracycline-inducible mCherry-GLUT1 into transfected cells. (b) Stable 293FT/Nluc-GLUT1 (control) or inducible 293FT/doxGB1 cells were induced for 48 hours with the indicated concentration of doxycycline (Dox). Cell lysates were harvested, deglycosylated and separated by SDS-PAGE prior to immunoblotting for GLUT1. All three species of GLUT1 can be detected on blots based upon their differential sizes. Two separate exposure times are shown to demonstrate the differential abundance of the transgenic GLUT1 fusion proteins, and the endogenous GLUT1. (c) Corrected BRET ratios were plotted relative to the concentration of doxycycline used to induce mCherry-GLUT1 expression. Data were log fit to a sigmoidal curve (R2 = 0.999), from which a doxycycline EC50 value of 4.69 +/− 0.25 ng/mL was determined. (d) The fluorescent intensity of each band on the immunoblot in (B) was normalized to actin to derive a corrected pixel value. These values were then expressed as fold increases relative to endogenous GLUT1 for each sample. Corrected BRET ratios were plotted against relative protein expression values for GLUT1, and fit to a sigmoidal curve as indicated (R2 = 0.996) to obtain an EC50 value of 3.01 +/− 0.10 fold expression relative to endogenous GLUT1 in 293FT cells. Error bars represent standard deviations of triplicate experimental replicates. (e) Model of higher order GLUT1 complexes represented with interactions between monomers. Each monomer is shown with its twelve alpha helical transmembrane domains, with extracellular (dashed) and intracellular (solid) loop regions. Our BRET data are consistent with previous studies indicating that GLUT1 transporters initially associate as N-to-N terminal dimers with contact interfaces between helicies 5 and 8 of one monomer and helicies 2 and 11 of the other. Two sets of dimers then associate into a tetramer via interfaces involving helix 9 from each monomer. This orientation limits further associations and specifies tetramers as the preferred species under saturation conditions.