Abstract
Pulque is a traditional Mexican alcoholic beverage produced from the fermentation of the fresh sap known as aguamiel (mead) extracted from several species of Agave (maguey) plants that grow in the Central Mexico plateau. Currently, pulque is produced, sold and consumed in popular districts of Mexico City and rural areas. The fermented product is a milky white, viscous, and slightly acidic liquid beverage with an alcohol content between 4 and 7° GL and history of consumption that dates back to pre-Hispanic times. In this contribution, we review the traditional pulque production process, including the microbiota involved in the biochemical changes that take place during aguamiel fermentation. We discuss the historical relevance and the benefits of pulque consumption, its chemical and nutritional properties, including the health benefits associated with diverse lactic acid bacteria with probiotic potential isolated from the beverage. Finally, we describe the actual status of pulque production as well as the social, scientific and technological challenges faced to preserve and improve the production of this ancestral beverage and Mexican cultural heritage.
Keywords: pulque, aguamiel, maguey, lactic acid bacteria, Saccharomyces cerevisiae, dextran, fructans, probiotics
Introduction
The role of maize in the origin of humans as described in the Popol Vuh, the sacred Maya book, together with the betrayal of the Toltec god Quetzalcoatl by Tezcatlipoca -the omnipresent god of the night who sees everything- are the two favorite stories of Mesoamerican mythology. Quetzalcoatl was ruined and had to exile after a ridicule behavior due to an excess of pulque intake. Both maize and pulque were key in the cosmological vision in Mesoamerica: while maize was linked to their origins, pulque was associated to their destiny, the Temoanchan, or lost Paradise, inhabited by several gods, where humans were created and pulque invented. Both Quetzalcoatl and Mayahuel -the Mexican nurturing mother- came to Earth to sing and dance to escape from paradise and to adopt the form of tree branches. However, they were punished by Mayahuel's grandmother who was a tzitzimitl -a darkness being- who, together with other tzitzimime destroyed the branch where Mayahuel was hiding. Quetzalcoatl, whose branch was not destroyed, buried Mayahuel with great sadness. The first agave plant grew in the place where Mayahuel was buried (Gonçalves de Lima, 1956; Anawalt, 1998; Ramírez, 2002).
However, the Agavaceae Family is very much older than pre-hispanic mythology, its origin dating back to about 10 million years ago (Good-Avila et al., 2006). Agave is a proliferous Family with nine known genera, comprising 300 species, most of them still present in Mexico. Agaves belong to the Amarilidaceas order and are endemic to Mexico. A restricted number of species are devoted to pulque including A. atrovirens, A. americana, A. salmiana, and A. mapisaga (Table 1; Alfaro Rojas et al., 2007; Mora-López et al., 2011).
Table 1.
Agave species used for aguamiel extraction and pulque production.
| Name | Accepted name according to the Plant List web sitea | Comments | References |
|---|---|---|---|
| A. atrovirens Kraw ex Salm-Dyck | Accepted | Cultured mainly in the states of Mexico, Tlaxcala, Hidalgo y Puebla | Alfaro Rojas et al., 2007 |
| A. atrovirens var. salmiana (Otto ex Salm-Dyck) Maire and Weiller | Synonym A. salmiana Otto ex Salm-Dyck | Cultured mainly in the states of Mexico, Tlaxcala, Hidalgo y Puebla | Alfaro Rojas et al., 2007 |
| A. americana L | Accepted | Cultured mainly in the states of Mexico, Tlaxcala, Hidalgo y Puebla | Alfaro Rojas et al., 2007 |
| A. mapisaga Trel | Accepted | Include 13 variants. Cultured mainly in the states of Mexico, Tlaxcala, Hidalgo y Puebla | Alfaro Rojas et al., 2007; Mora-López et al., 2011 |
| A. salmiana var angustifolia A. Berger | Accepted | Cultured mainly in the states of Mexico, Tlaxcala, Hidalgo y Puebla | Alfaro Rojas et al., 2007; Mora-López et al., 2011 |
| A. salmiana var ferox (K. Koch) Gentry| | Accepted | Include three variants | Mora-López et al., 2011 |
| A. salamina var salmiana | Unresolved name | The most diverse group including 31 variants | Mora-López et al., 2011 |
The Plant List (2010). Version 1.
The ancient Aztecs knew pulque as metoctli (from nahuatl language metl = agave or maguey, and octli = wine) agave wine, or iztacoctlli (from izac = white and octli = wine) white wine, or poliuhquioctli (from poliuhqui = spoiled or rotted and octli = wine) the spoiled beverage with unpleasant odor and flavor. It is probably from poliuhquioctli, that the Spanish conquerors designated as pulque, the freshly fermented agave beverage (Gonçalves de Lima, 1956; Sahagún, 1999). Pulque is a milky white, viscous, and slightly acidic beverage whit an alcoholic content which depends on several factors but usually between 4 and 7° GL, produced by spontaneous fermentation of aguamiel, the sugary sap extracted from the Agave species mentioned above (Secretaría de Economía, 1972b). According to Fray Bernardino de Sahagún, in his “Historia General de las Cosas de Nueva España,” numerous gods were involved in the Mayahuel's gift to humanity. Among others, he mentions Ometochtli who for the Aztecs was also the god of drunkenness, also associated with plant fertility and the wind. He ruled over the 400 Centzontochtli, or God rabbits of drunkenness, such as Patecatl, who knew how to mix aguamiel with plant roots, Cuatlapanqui (the “head- opener”) or Papaztac (the “nervous one”), among many others to whom the drunken and intoxicated were sacrificed (Gonçalves de Lima, 1956; Anawalt, 1998; Sahagún, 1999; Ramírez, 2002).
While most documents place the most probable origin of pulque in the ancient Otomi civilization toward the year 2000 BC, archeological evidence indicates that hunters and gatherers used maguey thousands of years ago (Jennings et al., 2005; Valadez-Blanco et al., 2012). Recent organic evidence shed new light on pulque history. In effect, although chemical components of this alcoholic beverage are water-soluble, limiting their conservation, hydrophobic lipids of food residues are more stable, Correa-Ascencio et al. (2014), applied a novel lipid biomarker approach to detect bacterial hopanoids derived from the widely recognized pulque fermenting bacteria Zymomonas mobilis as a pulque marker in more than 300 potsherds. The authors using this methodology were able to demonstrate for the first time the use of ceramic vessels to contain pulque in the locality of La Ventilla around 200–550 AD, at the height of Teotihuacan's culture. The presence of hopanes as bacterial markers of pulque, demonstrate that this beverage was produced in the ancient city of Teotihuacan and opens a new avenue of research for a systematic analysis to establish the level and intensity of pulque production and consumption in this culture (Correa-Ascencio et al., 2014).
During the height of the Aztec culture, pulque was produced and consumed preponderantly in religious and sacred rituals. It was restricted to the common citizens, with strict rules limiting its consumption. Excessive consumption was severely punished, in some cases including the capital punishment, even for priests. Upon the fall of the Aztec empire, pulque lost its religious significance gradually and became a food beverage and a popular intoxicant (Gonçalves de Lima, 1956; Ramírez et al., 2004; Ramírez Rodríguez, 2004). During the Spaniard Colony (1521–1821), pulque production was one of the main economic activities, and the most popular alcoholic beverage, resulting in the flourishment of Haciendas pulqueras (large farms dedicated to the cultivation of agave, pulque production, and commercialization), mainly in the central Mexican Plateau including the actual states of Hidalgo, Tlaxcala, Puebla, Morelos, Michoacán, and Querétaro. Interestingly, the production process remained practically unchanged since the Spaniard conquest and during Colony (Crist, 1939; Wilson and Pineda, 1963; Ramírez Rancaño, 2000). By 1629–1786, before the Mexican Independence War, pulque production and consumption was forbidden as it became a major health and social problems among the Indians. However, the economic relevance of maguey during the Spaniard Colony forced the authorities in 1786 to end the prohibition period as, despite the ban, pulque production competed with European wines and sugar cane liquor controlled by Spaniards (Lorenzo Monterrubio, 2007).
At the end of the Independence War (1810–1821), the production of pulque by the Haciendas pulqueras recovered its economic relevance, particularly by the introduction of the railway for the transport of thousands of liters of the fermented beverage directly from the Haciendas pulqueras to the main cities including Mexico City. By the beginning of the twentieth-century pulque production reached about 500 million L/year. By 1905, it is estimated that 350,000 L of pulque were consumed only in Mexico City. After the Revolution Civil War (1910–1920), the production structure of the Haciendas pulqueras was destroyed as pulque and its associated economic activity were owned by hacendados, an important part of to the upper class. By the period between 1920 to mid-1930s, the fresh pulque production and transport to Mexico City flourished again. However, by 1935–1940, the production and consumption of pulque was seriously affected again by an official anti-alcoholic policy, a severe devastation of agave plantations and the consolidation of the beer as a popular alcoholic beverage (Gonçalves de Lima, 1956; Ramírez Rancaño, 2000; Jácome, 2003; Ramírez et al., 2004; Ramírez Rodríguez, 2004; Lappe-Oliveras et al., 2008; Escalante et al., 2012).
Pulque had its major success in the last decades of the nineteenth century when rich fortunes derived from its successful production in haciendas and transport by train to the central Mexico urban centers. Significant efforts to preserve pulque and to face the increasing demand for beer failed. This effort, as well as the diversification of the agave industry, were led in particular by Ignacio Torres Adalid, known as “El Rey del Pulque” (“The King of Pulque” (Ramírez Rancaño, 2000). A campaign against pulque after the Mexican Revolution during the Venustiano Carranza government since 1914 until 1920, forced the hacendados to leave the country. Pulque consumption was associated with “criminality and degradation of the Mexican race.” That was the beginning of the pulque agroindustrial twentieth century debacle. Nevertheless, by 1882 pulque was the main alcoholic beverage consumed in the country and one of the most important Mexican agroindustries by the end of the nineteenth century. A train transported daily hundreds of wood barrels containing pulque from more than 300 haciendas and tinacales mainly from the Eastern states of Hidalgo and Tlaxcala, then rich region thanks to their “crops of the century” (maguey) and “white gold” (pulque) productivity (Parsons and Darling, 2000; Ramírez Rancaño, 2000; Jennings et al., 2005). Several factors have been mentioned to explain pulque's decline, among others the fact that pulque could not cope with the introduction of a competing alcoholic beverage: beer.
Despite the substantial differences in composition and organoleptic properties, probably the fact that pulque consumption dropped dramatically during the first decades of the twentieth century, besides the already mentioned campaign against consumption, was the lack of investment in science and technology. Interestingly, while consumers are now favoring traditional beers over the industrialized product, pulque consumers have no choice other than the traditional product which, in the context of the actual consumption trends, is now paradoxically, an advantage. The number of pulquerías offering pulque in Mexico City has considerably increased with more than 100 places offered to the consumer in internet pages, most of them of high quality (Ramírez Rancaño, 2000). The main production in Mexico is still the central state of Hidalgo where more than 260 million liters of pulque were produced in 2010, equivalent to 82% of the national production, followed by Tlaxcala with 13.3% and the State of Mexico with 2.68%, according to unofficial sources. As far as the National Institute of Statistics (INEGI), beer is described as responsible in 2014 of 1.2% of the total bulk manufacturing, while pulque was 0.0022% (INEGI, 2016). Other sources such as the “Encuesta Nacional de Adicciones 2011” (Instituto Nacional de Salud Pública, 2011) estimates that beer is consumed by 50 and 30% of the male and female population respectively, while other fermented beverages like pulque are consumed by only 4.4% of the population.
Traditional production of pulque
The main process of aguamiel extraction and pulque fermentation remains practically unchanged since pre-Hispanic times (Parsons and Darling, 2000; Jennings et al., 2005). Agave plants are relatively easy to cultivate as propagation is mainly carried by transplanting young off springs (called matecuates or hijuelos) from adult plants after a 7–25 years maturation cycle. Nevertheless, agave seeds cultivation has been an alternative for maguey propagation since pre-Hispanic times (Parsons and Darling, 2000). Agave plants are grown in specific agave plantations known as magueyeras where the trasplanted young matecuates are arrayed in parallel rows known as melgas or metepnatle (maguey wall) (Parsons and Darling, 2000; Ramírez Rancaño, 2000; Jácome, 2003). Agave plantations are located away from tall trees to avoid plant competence for light, water, and soil nutrients. Natural fertilization of agave plantations is self-provided by recycling naturally degraded agave plants or by the addition of agave ashes dispersed around the growing plants.
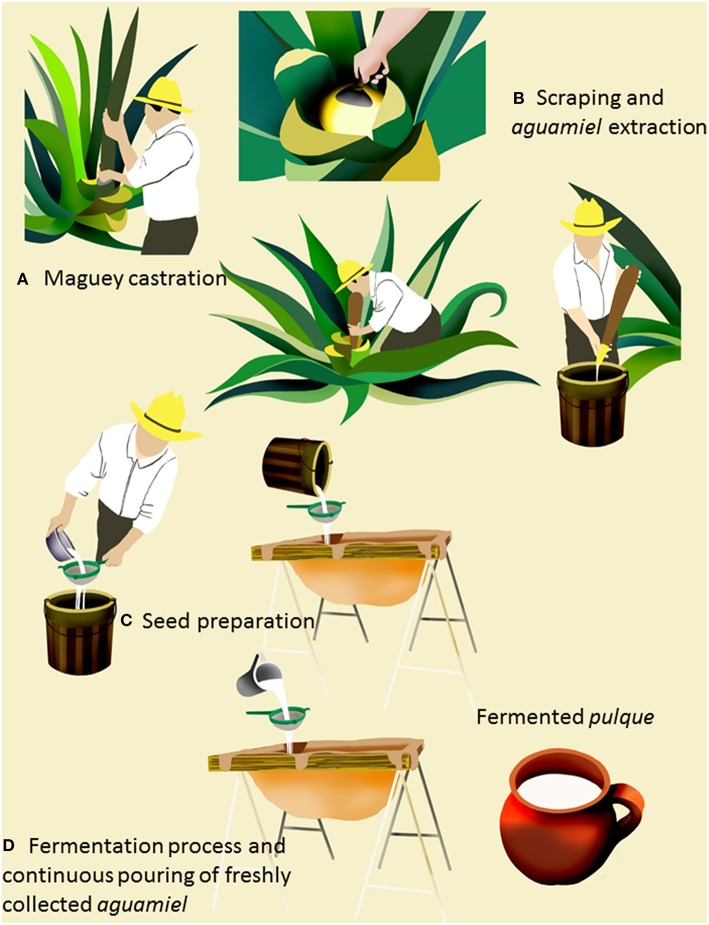
Aguamiel extraction and pulque elaboration are performed traditionally by the tlachiquero, who has a deep knowledge of the biology and care of the maguey species used for production. The process starts with the selection of mature plants from 6 to 15 years old and comprises four common steps with slight variations across producing zones (Crist, 1939; Wilson and Pineda, 1963; García-Garibay and López-Munguía, 1993; Parsons and Darling, 2000; Jennings et al., 2005): (1) castration, (2) pit scraping and aguamiel extraction, (3) seed preparation, and (4) fermentation (Figure 1).
Figure 1.
Traditional pulque elaboration process. The traditional process involves four common steps: (A) Castration of the mature plant by cutting the floral bud and make the pit (cajete). (B) Pit scraping to promote aguamiel accumulation and sap extraction. (C) Seed preparation. (D) Fermentation. For details of the castration process see Supplementary Files 1, 2.
Maguey castration
For this operation, selected mature plants are castrated by destroying the embryonic floral peduncle that surrounds the floral bud (quiote). During this operation, the central leaves of the plant (meloyote or heart), from which the flower rises are eliminated using a pointed and sharp instrument, leaving a cavity (known as cajete) in the center of the plant (Jennings et al., 2005). The cavity is covered with a large stone or with agave leaves to protect it from animals and the environmental conditions. A maturation period follows castration and varies from 3 months to 1 year (Crist, 1939; Wilson and Pineda, 1963; García-Garibay and López-Munguía, 1993; Parsons and Darling, 2000; Jennings et al., 2005).
The castration process varies among producing regions: in the production region of Huitzilac (Morelos state), the cavity is digged without eliminating the central leaves, and the floral bud is cut off after the maturation process. The precise moment for castration is the thachiquero responsibility to avoid floral budding. If the inflorescence grows, the plant will never produce aguamiel. Moreover, early castration will result in a reduced volume of poor quality aguamiel production. Traditionally, some hints used by the tlachiquero to select mature plants are the abundance of leaves, the thinness of meloyote, and the surrounding leaves, which are also spikeless and adopt a lighter green tone. A detailed video showing the castration process and the instruments used is available in Supplementary Files 1, 2 (Crist, 1939; Wilson and Pineda, 1963; García-Garibay and López-Munguía, 1993; Parsons and Darling, 2000; Jennings et al., 2005).
Scraping and aguamiel extraction
Fresh aguamiel is a lightly cloudy, thick, very sweet, fresh-plant flavored and neutral to slightly acid sap. By scraping the cajete's wall the sap outflow is induced, so aguamiel flows and accumulates in the cavity. This operation is performed by the tlachiquero using a scraping tool (Crist, 1939; Wilson and Pineda, 1963; García-Garibay and López-Munguía, 1993; Parsons and Darling, 2000; Jennings et al., 2005). The accumulated sap is extracted twice a day (usually at daybreak and dusk) by oral suction using a dried gourd (Lagenaria siceraria) known as acocote. After each aguamiel collection, the walls of the cavity are scraped again to maintain the sap flow induction. Freshly collected aguamiel is stored in plastic containers and transported to specific vats where the main fermentation takes place (Figure 2). A mature agave plant may produce aguamiel from 3 to 6 months until the plant dies, depending on the frequency of the scraping process. On a daily basis, the plant yields 4–6 L of aguamiel with a maximum average production of around 1000 L in its production lifetime (Crist, 1939; Wilson and Pineda, 1963; García-Garibay and López-Munguía, 1993; Parsons and Darling, 2000; Ramírez Rancaño, 2000; Jennings et al., 2005).
Figure 2.
Aguamiel extraction from producing maguey, transportation to the tinacal and fermentation process. (A) Tlachiquero extracting freshly aguamiel with an acocote (Hidalgo state). (B) Aguamiel is transferred into a plastic container for transportation to the tinacal (Morelos state). (C) Freshly collected aguamiel appearance (Morelos state). (D) Aguamiel accumulated in cajete previous to the twice-daily extraction (Hidalgo state). (E) Aguamiel pouring into a plastic vat for seed preparation (Hidalgo state). (F) Fermented pulque in a plastic vat (Hidalgo state). (G) Fermented pulque in a traditional leather vat (Hidalgo state). (H) Serving pulque for direct consumption from the fermentation vat (Tlaxcala state). Note the characteristic filament associated to final product viscosity.
Seed preparation
This operation refers to the production of starting material (inoculum) for the fermentation of freshly collected sap in a new container. For this purpose, around 2 L of fermented pulque are placed in a ~20 L vat made of clay, glass, wood, plastic or fiberglass, were fresh, high-quality aguamiel is poured. A spontaneous fermentation starts at room temperature until a characteristic alcoholic, and acetic taste develops or until a white layer -called zurrón- is formed on the surface, a process that usually takes from 1 to 4 weeks, depending on the season). Finally, the tlachiquero transfers the fermented product (seed) to one or more clean vats where pulque fermentation will takes place once freshly collected aguamiel is added (Crist, 1939; García-Garibay and López-Munguía, 1993; Parsons and Darling, 2000; Jennings et al., 2005; Escalante et al., 2012).
Pulque fermentation
Fermentation takes place in vats usually made of cow-leather, glass-fiber, plastic or wood barrels located either in closed rooms known as tinacal or in specific open spaces (Figure 2). Freshly collected aguamiel is filtered to separate insects or any large object and poured into the vat, where the seed was previously transferred. The fermentation time varies strongly depending on aguamiel quality, seed maturity, season and producing region, among other factors. It usually lasts from 3 to 6 h, but overnight or even extended periods of time (e.g., 3–12 days) are not uncommon (Crist, 1939; Parsons and Darling, 2000; Ramírez Rancaño, 2000; Jennings et al., 2005).
Mexican norm NMX-V-022.1972 defines the sensorial properties required for the fresh collected sap or aguamiel used for pulque fermentation as a translucent, light amber-colored, sweet, fresh-flavored and lightly acid liquid with characteristic flavor and odor. Based on their physicochemical properties this norm defines two types of aguamiel. Type I or high-quality aguamiel and Type II, poor quality or slightly acid aguamiel. As for the alcohol content, Mexican norm NMX-V-037-1972 defines the alcoholic content of pulque. According to this norm, pulque is a beverage with low alcoholic content, not-clarified, of white color, acid, and viscous texture. The norm defines two types of pulque, Type I or pulque for seed (Section Biochemistry of the Fermentation) and “puntas” and Type II or commercial pulque. The requirements specified for aguamiel and pulque in norms NMX-V-022.1972 and NMX-V-037-1972 are presented in Table (Secretaría de Economía, 1972a,b).
Despite the Mexican norm NMX-V-037-1972 defined the desirable physicochemical properties of bulk pulque for direct consumption, particularly for density, pH (3.5–4.2), and alcohol degree (4–9%) (Table 2; Secretaría de Economía, 1972b), during traditional production of pulque the degree of fermentation varies according to the producer and is considered adequate when a characteristic alcohol, acetic notes, and texture (viscosity) is reached. Fermented pulque is withdrawn from the vat and consumed either natural or curado, as it is known when mixed with macerated fruits, vegetables, nuts or spices (Parsons and Darling, 2000; Ramírez Rancaño, 2000; Jennings et al., 2005; Lappe-Oliveras et al., 2008; Escalante et al., 2012). Sometimes, particularly when the fermentation yields a low-quality pulque (e.g., with low viscosity or off flavors), the tlachiquero adds plant roots, herbs or pieces of agave plants, a practice known as cardón, to improve the fermentation process (Parsons and Darling, 2000; Jennings et al., 2005).
Table 2.
Physicochemical characteristics of aguamiel and pulque.
| Characteristic | Aguamiel | References | |||
|---|---|---|---|---|---|
| Type I | Type II | ||||
| Minimum | Maximum | Lower to | |||
| pH | 6.6 | 7.5 | 4.5 | Secretaría de Economía, 1972a | |
| Density (°Bé) | 5 | 7 | 4.5 | ||
| Refractive index (immersion, 20°C) | 59 | 100 | 27 | ||
| Total solidsa | 13 | 17 | 7 | ||
| Total reducing sugarsa (as glucose) | 8 | 12 | 6 | ||
| Direct reducing sugarsa (as glucose) | 2 | 3 | 3 | ||
| Gumsa (as glucose) | 2 | 6 | 0.2 | ||
| Proteinsa | 300 | 600 | 100 | ||
| Ashesa | 300 | 430 | 100 | ||
| Total aciditya (as lactic acid) | 0.9 | 1.03 | 4 | ||
| Pulque | |||||
| Type I | Type II | ||||
| Minimum | Maximum | Minimum | Maximum | ||
| Refractive index (immersion, 20°C) | 32 | 35 | 25 | ND | Secretaría de Economía, 1972b |
| Refractive index (Abbé, 20°C) | 1.3390 | 1.3406 | 1.3365 | 1.3380 | |
| pH | >3.7 | 4.2 | 3.5 | 4 | |
| Total aciditya (as lactic acid) | 0.4 | 0.75 | 0.4 | 0.7 | |
| Total reducing sugarsa (as glucose) | 0.1 | 0.8 | 0.2 | 0.5 | |
| Alcoholic degree (%/vol) | 6 | 9 | 4 | 6 | |
mg/100 mL, ND, non-defined. °Bé, Baumé degrees.
Microbiology and biochemistry of the fermentation
Toward the definition of an essential microbiota responsible for pulque fermentation
Pulque fermentation is a batch non-stirred process, performed under non-aseptic conditions. The microorganisms involved in the fermentation are those naturally occurring during sap accumulation in the cajete cavity in maguey and those incorporated during collection, transport, seed preparation and manipulation (Lappe-Oliveras et al., 2008; Escalante et al., 2012). Earlier studies on the microbiology of pulque performed by Sanchéz-Marroquín by 1950's reported the presence of homo- and heterofermentative LAB identified as Lactobacillus sp., Leuconostoc mesenteroides, and L. dextranicum, the yeast Saccharomyces cerevisiae (identified as S. carbajali) and the α-Proteobacteria Zymomonas mobilis (identified as Pseudomonas lindneri) (Sánchez-Marroquín and Hope, 1953).
These microorganisms develop three distinctive metabolic products during pulque fermentation: lactic acid produced by Lactobacillus sp. and Leuconostoc sp. which conduct the acid fermentation, ethanol resulting from the alcoholic fermentation and synthesized mainly by S. cerevisiae and Z. mobilis, and the extracellular polysaccharides (EPS), which include dextrans and fructans produced from sucrose by glycosyltransferases from Leuconostoc sp. and Z. mobilis (Sánchez-Marroquín and Hope, 1953; Lappe-Oliveras et al., 2008; Escalante et al., 2012). Due to this complex fermentation process, pulque is considered an acid and viscous alcoholic beverage. Sánchez-Marroquín et al. (1957), used isolated strains of the species mentioned above in a mixed inoculum, as a starter for a controlled fermentation of aguamiel. The Sánchez-Marroquín group was able to obtain a fermented beverage with similar organoleptic and physicochemical characteristics of the fermented product regarding flavor, aroma, alcohol content, acidity, and viscosity, suggesting the essential role of these microorganisms in traditional pulque properties (Sánchez-Marroquín et al., 1957).
Further studies on the microbiology of pulque, allowed the identification of a wider bacterial and yeast diversity. This diversity has been classified according to the microorganisms' main metabolic traits as (i) acid producing bacteria, including LAB and acetic acid bacteria (AAB); (ii) alcohol-producing microorganisms, including S. cerevisiae and Z. mobilis, (iii) dextran-producing bacteria (L. mesenteroides), and (iv) putrefactive microorganisms (Table 2). Interestingly, microorganisms involved in the four fermentative processes of pulque fermentation have been systematically isolated in pulque samples of different regions around the central Mexican Plateau (Escalante et al., 2004; Lappe-Oliveras et al., 2008). Regarding yeast diversity in pulque, Saccharomyces, and non-Saccharomyces species have been identified and proposed as essential fermentative yeast responsible for the production of ethanol, amino acids, vitamins, and volatile flavor compounds participating in the sensorial properties of the beverage (Lappe-Oliveras et al., 2008). Additionally, diverse killer and killer-resistant yeasts were isolated from aguamiel and pulque, some of them with a remarkable alcohol tolerance (Estrada-Godina et al., 2001) (Table 2).
Analysis of the bacterial diversity of pulque samples of different geographical origins (Estado de Mexico, Hidalgo, and Morelos states) as determined by 16S rDNA clone libraries was reported by Escalante et al. (2004). These authors reported the identification of an even wider diversity including non-previously reported bacteria. Interestingly, this study allowed to conclude that the bacterial diversity present among pulque samples was dominated by LAB, particularly Lactobacillus acidophilus (homofermentative LAB), corresponding to ~60–85% of total 16S rDNA clones analyzed for each pulque sample. Other clones identified as L. mesenteroides ranging from ~0.5 to 25% of total clones analyzed for each sample. Z. mobilis was detected in low amounts only in two samples, and 16S rDNA clones identified as the AAB Acetobacter pomorium and Gluconobacter oxydans (~33% of detected clones) were detected only in one sample. These results allowed defining the common bacterial diversity in pulque samples of different geographical origin, as well as a bacterial diversity specific of a given region (Escalante et al., 2004).
Assessment of the changes in the bacterial community during the fermentation of pulque
The dynamics of bacterial diversity was studied in the laboratory with fresh aguamiel and pulque collected from Huitzilac, Morelos state by Escalante et al. (2008), using a polyphasic approach, including the isolation of LAB, aerobic mesophiles, and 16S rDNA clone libraries from total DNA extracted from fresh collected aguamiel used as substrate, after inoculation with previously produced pulque and followed by 6-h fermentation. Freshly collected aguamiel contained a count of 1.3 × 107 CFU/mL of total aerobic mesophilic bacteria (AMB), 3.2 × 109 CFU/mL of total LAB, and 3.1 × 104 CFU/mL of total yeasts. These results revealed the presence of a major microbial content associated to the accumulated sap in the maguey cavity (Escalante et al., 2008).
These authors also reported that total microbial counts determined after mixing fermented pulque with freshly collected aguamiel (initial fermentation time = 0 h) resulted in an increase of yeasts to 8.8 × 106 CFU/ml. After three h of fermentation, total yeasts further rose to 1.4 × 107 CFU/mL and remained constant until the end of the fermentation (1.9 × 107 CFU/mL). Total counts of both bacterial groups at the beginning of the fermentation were 1.2 × 107 CFU/mL for total AMB and 1.5 × 108 CFU/mL for LAB. By the end of the fermentation, total counts of both bacterial groups remained relatively constant as reached 3.5 × 107 CFU/mL and 1.5 × 108 CFU/mL, respectively (Escalante et al., 2008).
The microbial diversity identified in aguamiel was composed mainly by LAB including L. mesenteroides, L. kimchi, L. citreum and in minor proportion Lactococcus lactis. The γ-Proteobacteria Erwinia rapontici, Enterobacter sp., and Acinetobacter radioresistens were the second most abundant bacterial group detected in agave sap. As the identified γ-Proteobacteria are naturally distributed microorganisms in diverse environments such as freshwater, soil, and vegetable surfaces, it may be possible to suppose that these bacteria are a contaminant incorporated to the sap during its accumulation in the cajete, or during the extraction and handling procedures (Escalante et al., 2008). Although Escalante et al. (2008) did not report the detection of lactobacilli in aguamiel, the isolation of Lactobacillus brevis and L. collinoides from agave sap samples collected from Huitzilac, Morelos state, was described in a recent publication (Reyes-Naya et al., 2016).
The addition of freshly collected aguamiel to previously fermented pulque results in a considerable increase in the count of yeasts (~155% on total CFU/mL respect aguamiel). L. kimchi and A. radioresistens decreased, and L. mesenteroides remained relatively constant respect aguamiel (Escalante et al., 2008). Interestingly, after mixing aguamiel with pulque (T0), the most abundant microorganism detected was the LAB identified as Lactobacillus acidophilus. The γ-Proteobacteria Enterobacter agglomerans, and the α-Proteobacteria Z. mobilis and Acetobacter malorum were also detected but in low proportions in T0. Important physicochemical changes were observed in T0. After mixing fresh aguamiel and fermented pulque, the pH decreased from 6.0 to 4.5 in the mixture. Total sugars in aguamiel decreased 53.9%, and total carbon in fermented products detected in T0 (mainly as ethanol) increased 942.5% when compared to aguamiel (Escalante et al., 2008; Figure 3).
Figure 3.
Microbial, metabolic and physicochemical changes during pulque fermentation. Proposed microbial, physicochemical and metabolic changes during pulque fermentation as described by Escalante et al. (2008). (A) Total CFU/mL counts for yeasts; (B) Total mesophilic aerobes (TMA); (C) LAB determined during 6 h fermentation in laboratory; (D) Sugar consumption expressed as mM hexose equivalent; (E) Fermentation products (ethanol, lactic acid, and acetic acid) expressed as mM C; (F) Cultivable diversity (% of four most abundant isolates); (G) Culture-independent diversity (% of four most abundant 16S rDNA clones); (H) Scanning electron micrograph corresponding to pulque fermentation after 6 h showing some yeast and short cocci chains (T6) (non-previously published photograph); (I) Aguamiel accumulated in cajete; (J) Fermented pulque. AM, aguamiel, T0, T3, and T6, the start of the fermentation, 3 and 6 h of cultivation, respectively. Ama, Acetobacter malorum; Ara, Acinetobacter radioresistens; Eag, Enterobacter aglomerans; Erh, Erwinia rhapontici; Ent, Enterobacter sp.; Kas, Kluyvera ascorbata; Lbh, homofermentative Lactobacillus sp.; Lbs. Lactobacillus sp.; Lac, L. acidophilus; Lla, Lactococcus lactis; Lme, Leuconostoc mesenteroides; Lci, L. citreum; Lki, L. kimchi; Sce, Saccharomyces cerevisiae; Zmo, Zymomonas mobilis; Uba, Uncultured bacterial clone.
Microbial diversity present at T0 includes microorganisms in aguamiel and those from fermented pulque resulting in a microbial diversity composed by homo- and heterofermentative LAB, EPS-producing LAB, AAB, AMB, ethanol producing Z. mobilis, and yeasts. After 3 h of fermentation, diverse changes in the microbial diversity occurred despite the relatively constant total CFU/ml observed for LAB and total AMB. L. acidophilus, L. mesenteroides, and E. agglomerans were the most abundant bacteria; some others (both LAB and Proteobacteria) decreased or disappeared while yeast increased 102.9%. Also after 3 h, total sugars measured in T0 decreased 56%, and total carbon in fermented products (mainly ethanol) increased 120.7%. Finally, after 6 h of fermentation, the final microbial diversity was composed mostly by the homofermentative L. acidophilus, L. mesenteroides. L. lactis subsp. lactis and the α-Proteobacteria A. malorum. As a consequence of the microbial activity, after 6 h of fermentation, the final pH further decreased to 4.3, while 63.3% of the total sugar present after inoculation was consumed. Final fermentative products corresponded to 939.5 mM C as ethanol, 106.2 mM C as acetic acid, and 108 mM as lactic acid (Figure 3; Escalante et al., 2008).
Biochemistry of the fermentation
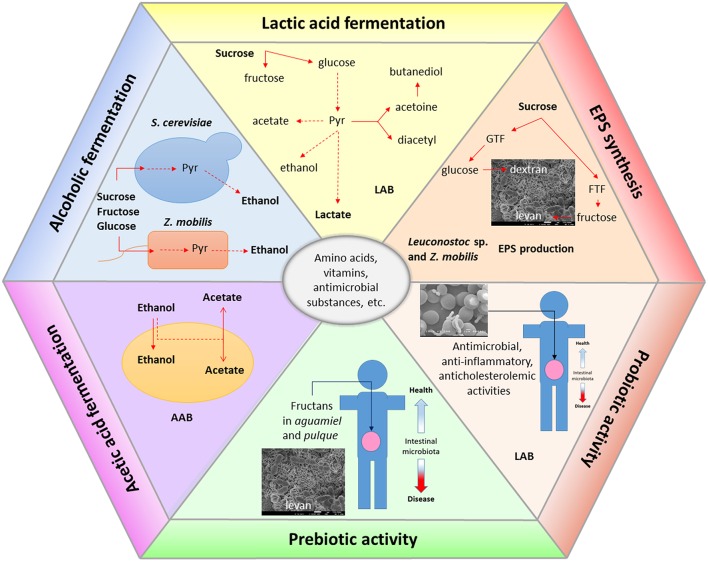
As already described, microbiological studies of aguamiel and pulque have revealed the presence of a complex bacterial and yeast diversity. The final sensorial properties of pulque are defined by the simultaneous development of the four fermentation types already described in Section Toward the Definition of an Essential Microbiota Responsible for Pulque Fermentation, which depend on the most abundant microorganisms present in pulque, also depending on its geographical origin (Figure 4):
Figure 4.
Metabolic traits of main microbial groups present in aguamiel and during pulque fermentation. Main metabolic traits comprise homo- and heterofermentative lactic acid metabolism by LAB. Production of ethanol by Saccharomyces, non-Saccharomyces yeasts, and Z. mobilis. Acetic acid metabolism. Extracellular polysaccharide synthesis resulting in the synthesis of dextran and levan polymers by Leuconostoc sp. and Z. mobilis (levan). Microorganisms and metabolic pathways involved in the amino acid production, vitamins, and some antimicrobial compounds remain to be determined. Functional properties such as prebiotic and probiotic activities are related to fructooligosaccharide content in aguamiel and pulque or produced by LAB such as Leuconostoc sp. Probiotic properties are related to diverse LAB identified as Lactobacillus sp. and Leuconostoc sp.
An acid fermentation performed mainly by homo- and heterofermentative LAB such as Lactobacillus and Leuconostoc (Sánchez-Marroquín and Hope, 1953; Sánchez-Marroquín et al., 1957; Escalante et al., 2004, 2008; Lappe-Oliveras et al., 2008), species involving the catabolism of available glucose to pyruvate by the Embden-Meyerhoff pathway and its subsequent conversion to lactic acid and other metabolic products such acetic acid, CO2, and ethanol (Carr et al., 2002).
An alcoholic fermentation performed mainly by the yeast S. cerevisiae and in minor degree by the α-Proteobacteria Z. mobilis from sucrose, glucose, and fructose in aguamiel. Z. mobilis converts efficiently fermentable sugars to ethanol and CO2 by the Entner-Doudoroff pathway (Lau et al., 2010; Xiong He et al., 2014).
The synthesis of EPS performed by Leuconostoc species including L. mesenteroides and L. kimchi resulting in the production of dextran and fructan exopolysaccharides from sucrose by enzymes such as glucosyl- and fructosyl-transferases, respectively (Chellapandian et al., 1998; Torres-Rodríguez et al., 2014). Z. mobilis is also a levan producer (Xiong He et al., 2014).
An acetic acid fermentation performed probably by AAB such Acetobacter and Gluconobacter species (Escalante et al., 2004, 2008). AAB produce acetic acid as the main product through the oxidation of sugars, sugar-alcohols, and ethanol by the sequential activity of alcohol dehydrogenase and aldehyde dehydrogenase located in the outer membrane. G. oxydans catabolizes preferentially sugars and Acetobacter sp. in a minor proportion. Additionally, these bacteria produce gluconic acid and oxidize several organic acids including lactic acid to CO2 and water (Raspor and Goranovič, 2008).
The specific role of diverse microorganisms, particularly those identified as dominant in aguamiel and pulque fermentation in the production of essential amino acids, vitamins, and a variety of flavored volatile compounds remains a research subject (Figure 4).
Functional properties of aguamiel and pulque
Nutritional benefits associated with pulque consumption
According to the traditional pharmacopeia, aguamiel and pulque consumption has been related to diverse nutritional and health-promoting benefits since Pre-Hispanic times despite the alcohol content of the fermented beverage (mild value ~4.8% ethanol) (Secretaría de Economía, 1972b; Backstrand et al., 2002). However, the first study directly reporting the health benefits of pulque consumption, is the successful treatment of scurvy in penitentiary inmates in 1887 in Puebla state, well before the discovery of vitamin C (Ramírez Rancaño, 2000). The first systematic study on the nutritional benefits of pulque consumption associated with a regular intake was carried out in the indigenous Otomí population of theValle del Mezquital (Hidalgo state) was performed by Anderson et al. (1946). Results obtained from the analysis to 100 adult consumers, under a 7 days' based diet, conclude that daily intake of pulque (up to 2 L) provides calories (12%), total protein (6%), thiamin (10%), riboflavin (24%), niacin (23%), vitamin C (48%), calcium (8%), and iron (51%). These results indicate that for this ethnic group, pulque consumption constitutes the second most important “food” in the diet after tortilla. Authors concluded that these results are relevant considering the marginal character of this indigenous population diet, highlighting the daily contribution of vitamin C trough pulque (Anderson et al., 1946).
Sánchez-Marroquín and Hope (1953), determined the main content of some vitamins in pulque (μg/100 mL of pulque) and found: 65.2 of pantothenic acid, 30.7 of thiamine, 21.6 of ρ-amino benzoic acid, 23 of pyridoxine, including also 19.6 (ng/100 mL of pulque) of biotin (Sánchez-Marroquín and Hope, 1953). Further studies on the nutritional benefits of pulque intake demonstrated that after maize tortillas and legumes, pulque was the third most important source of iron (non-heme form), ascorbic acid, riboflavin, and other B-vitamins. Additionally, pulque provides significant amounts of folate, steroidal saponins, many of them bioactive (Backstrand et al., 2002). Furthermore, pulque is a source of phytase which has been proposed to be produced by Lactobacillus species and S. cerevisiae present in pulque, resulting in an increased bioavailability of iron and zinc present in maize (Tovar et al., 2008). Regarding the amino acids content, it was found that pulque contains 0.27 g/100 pulque of crude protein. Detected amino acids (g/16 g of N), included Ile (4.04), Leu (8.65), Lys (1.76), Cys (1.59), Phe (6.45), Tyr (2.76), Thr (4.21), Trp (2.35), Val (5.12), and His (2.01) (Morales de León et al., 2005). The total content of protein and amino acids is substantially less than what the common myth in rural areas propose, which is that “pulque lacks one degree to have the benefits of meat.”
Studies on the relationship of iron status in a rural population from central Mexico highlands (Valle de Solís), performed in 125 non-pregnant women aged between 16 and 44 years old, assessed food intake during 12 months. Iron status determined after blood analysis showed higher plasma ferritin concentrations associated with significant intakes of non-heme iron and ascorbic acid. This study showed that better iron status correlated with significant pulque intake, an important source of non-heme iron and ascorbic acid, influencing the iron status of women from this rural zone. In this study, daily ethanol intake by pulque consumption was calculated using an average content of 47 g ethanol/L pulque; wich corresponds to the mean between 29 and 65 g/L (Backstrand et al., 2002).
The study of pulque intake in 70 expectant mothers from the Valle de Solís showed that 72.9% of women included in the study consumed pulque during pregnancy, and 75% continued consumption during the postpartum period as an important source of nutrients and energy. The consumption of 0.5 L of pulque, the amount commonly consumed by women in the research site supplied 24 g of ethanol, 9% of energy, 42.9% of ascorbic acid, 6.7 of thiamine, 5.9% of riboflavin, and 14.6% of iron of the Mexican Recommended Dietary Intake (RDI) during pregnancy. Results indicated that ascorbic acid intake from pulque was associated with a decrease in the risk of low ferritin and hemoglobin levels. The ethanol content in pulque was proposed to enhance iron absorption and to improve mother's daily iron intake. These authors showed the association between pulque intake during lactation and robust newborn growth, suggesting a beneficial effect of low pulque intake associated probably to the micronutrient content of the beverage. However, the study concludes that earlier intake of pulque during pregnancy and lactation was associated with poorer child height and weight (Backstrand et al., 2001, 2004).
Aguamiel nutritional content and possible functional properties
Regarding aguamiel, the sap collected from A. salmiana ‘Gentry’ contains low amounts of crude fiber (0.57%), crude protein (0.69%) and a high level of nitrogen free extract (98.1%, corresponding to highly digestible carbohydrates). Mineral content analysis showed (in mg/L of aguamiel) 100 of N, 200 of Ca, 200 of P, 200 of Mg, 21.5 of Fe, 14.1 of Zn, 7.4 of Cu, and 19.9 of B. The consumption of 850 mL of aguamiel satisfy the daily human requirements of Fe and Zn, according to the Recommended Dietary Allowances or Adequate Intake (Silos-Espino et al., 2007).
The sap collected from A. mapisaga ‘Blanco’ contains (wt % in dry matter) 11.5% composed mainly of 75% of sugars (sucrose, fructose, glucose, and fructooligosaccharides), 0.3% of free amino acids (essential amino acids with exception of methionine), 3% of proteins, and 3% of ashes. Besides essential amino acid 26 mg/L of aguamiel of γ-aminobutyric acid (GABA) were identified (Ortiz-Basurto et al., 2008). These authors determined that aguamiel composition remain relatively stable throughout the production period (5 months), suggesting that the sap produced by A. mapisaga could be a stable substrate for a standardized pulque production processes.
Agave plants possess branched fructans (graminan) and graminan neoseries with two branches. One branch is attached to the fructosyl residue while the other is attached to the glucosyl unit of the sucrose molecule. These fructans have been designated as agavins, which are inulins with a complex mixture of structures and different degree of polymerization (DP) (Velázquez-Martínez et al., 2014). Due to the high fructan and fructooligosaccharide (FOS) content, agave extracts as well as the sap (consumed directly or concentrated) from different species, have been considered as an alternative source for prebiotic FOS syrups. This type of food additives has received increased attention due to its low glycemic index and, their demonstrated beneficial health effects such as improving calcium absorption in postmenopausal women, iron absorption, and, colon cancer prevention (García-Aguirre et al., 2009; Santos-Zea et al., 2016). Aguamiel from A. mapisaga “Blanco” contains inuline-type fructans (10.2% wt in dry matter) and glucooligosaccharides. The fructooligosaccharides identified up to now are highly branched, containing β-fructosyl units linked mainly by β1 → 2, but also β2 → 6 linkages (Ortiz-Basurto et al., 2008). Different extracts of A. angustifolia “Haw” agave have high molecular weight and branched fructans with the same structure regarding fructan linkages but different DP: high (3–60 fructose units), medium (2–40), and low (2–22) (Velázquez-Martínez et al., 2014).
Agave fructooligosaccharides have a demonstrated prebiotic function. In effect, several reports have demonstrated the in vitro growth promoting effects of diverse lactobacilli and bifidobacteria and well-known probiotic strains including L. acidophilus, B. lactis, B. infantis, B. animals, and B. adolescentis, some of them considered as predominant in human intestinal microbiota (Tripathi and Giri, 2014; Velázquez-Martínez et al., 2014; Castro-Zavala et al., 2015). As discussed above, aguamiel and pulque possess diverse well-documented nutritional traits; the main disadvantage of pulque remains its alcoholic content, which limits and restricts its promotion and consumption (Narro-Robles and Gutiérrez-Avila, 1997; Backstrand et al., 2001, 2004).
Assessment of the probiotic potential of LAB isolated from aguamiel and fermented pulque
The isolation and assessment of the probiotic potential of LAB from non-dairy products for the formulation of health-promoting functional foods have been a trending activity (Tripathi and Giri, 2014). This type of products containing probiotic bacterial strains but based on juices, fruits, and cereals, offer significant advantages as an alternative to dairy-based functional products such as low cholesterol and the absence of dairy-allergenic substances (Soccol et al., 2012).
LAB detected as the most abundant bacteria in pulque such as Lactobacillus acidophilus and L. plantarum (Table 3), are proposed to play an important role also due to their antimicrobial activities. The natural resistance of these LAB to the final pulque pH and alcohol content, their abundance at the end of fermentation (Escalante et al., 2008), and the traditional application of pulque for the treatment of gastrointestinal diseases suggest that LAB involved in pulque fermentation are potential probiotic candidates.
Table 3.
Microbial diversity detected in aguamiel and during pulque fermentation.
| Bacteria | Yeasts/Fungi | Remarkable metabolic traits defining sensorial properties of aguamiel or pulque | References |
|---|---|---|---|
| Lactobacillus sp. Leuconostoc mesenteroides, L. dextranicum Zymomonas mobilis | Saccharomyces cerevisiae | Essential microorganisms responsible for acid (lactic acid), alcoholic and production of EPS | Sánchez-Marroquín and Hope, 1953; Sánchez-Marroquín et al., 1957 |
| Yeasts isolated from aguamiel: Candida lusitaneae, Klyuveromyces marxianus var bulagricus (+), S. cerevisiae Yeast isolated from pulque: C. valida (+), S. cerevisiae (chevalieri), S. cerevisiae (capensis), K. marxianus var lactis (+) | Several isolates of C. valida, S. cerevisiase (chevalier) isolated from pulque were able to resist to >10% of alcohol. Potential relevance in ethanol production during the fermentation and resistance to killer toxins | Estrada-Godina et al., 2001 | |
| Acetobacter aceti, A. aceti subsp. xylinus, Bacillus simplex, B. subtilis, Cellulomonas sp., Escherichia sp., Kokuria rosea, Lactobacillus sp., L. delbrueckii, L. vermiforme, Leuconostoc sp., L. mesenteroides subsp. dextranicum, L. mesenteroides subsp. mesenteroides, Macrococcus caseolyticus, Micrococcus luteus, Sarcina sp., Z. mobilis subsp. mobilis | Cryptococcus sp., Candida parapsilosis, Clavispora lusitaniae, Debaryomyces carsonii, Hanseniaspora uvarum, Kluyveromyces lactis, K. marxianus, Geotrichum candidum, Pichia sp., P. guilliermondii, P. membranifaciens, Rhodotorula sp., R. mucilaginosa, Saccharomyces bayanus, S. cerevisiae, S. pastorianus, Torulaspora delbrueckii | Essential microorganisms responsible for lactic and acetic fermentation (LAB and acetic acid bacteria), alcoholic fermentation (Z. mobilis and S. cerevisase), EPS production y (Leucocnostoc sp.) and putrefactive bacteria | Lappe-Oliveras et al., 2008 |
| Analysis of 16S rDNA clone libraries allowed to identify Lactobacillus acidophilus, L. kefir, L. acetotolerans, L. hilgardii, L. plantarum, Leuconostoc mesenteroides subsp. mesenteroides, L. pseudomesenteroides, Acetobacter pomorum, Gluconobacter oxydans, Zymomonas mobilis, Flavobacterium jhonsonae, Hafnia alvei | Homofermentative L. acidophilus was identified as the most abundant microorganism in three analyzed samples from different geographical origin, suggesting a possible essential role in lactic acid fermentation. L. mesenteroides was present in low proportion respect lactobacilli. Z. mobilis and AAB were detected low percentage or absent. Presence of possible putrefactive or contaminant bacteria | Escalante et al., 2004 | |
| A combined culture dependent and 16S rDNA libraries approach allowed to identify those microorganisms present in freshly collected aguamiel and during a 6 h of fermentation. α-Proteobaceria: Acetobacter maloruma, A. orientalisb, Z. mobilis subsp. pomaceaeb, γ-Proteobacteria: Citrobacter sp., Enterobacter sp.a, E. agglomeransa, Erwinia rhaponticia, Kuyvera acorbatac, K. cochleaea, Providencia sp.a, Serratia grimensiia, Acinetobacter radioresistensb, Sterotrophomonas sp.a, Chryseobacterium sp. Firmicutes: Bacillus sp.a, B. licheniformisa, Lactobacillus sp.c, L. acidophilusb, L. hilgardiib, L. paracollinoidesb, L. sanfranciscensisb, Lactocoocus sp.a, L. lactisa, L. lactis susp. lactisa Leuconostoc kimchic, L. citreumc, L. gasocomitatumb, L. mesenteroidesc, L. pseudomesenteroidesc, Pediococcus urinaeequia, Streptococcus devieseia | S. cerevisiaeb | Leuconostoc citreum and L. kimchi species were identified as the most abundant LAB in aguamiel. After mixing fresh aguamiel with previously fermented pulque, L. acidophilus, L. mesenteroides were the most abundant LAB during 6 h of fermentation. E. agglomerans was the most abundant non-LAB during the first 3 h of fermentation. Z. mobilis and AAB were absent in aguamiel but detected in low proportion during the fermentation process Total bacterial counts (CFU/mL) for LAB and total aerobic mesophilic bacteria were constant during 6 h of fermentation. Total yeast counts (CFU/mL) detected in aguamiel increased after mixing aguamiel with fermented pulque, increased until 3 h and maintained constant until the end of the fermentation | Escalante et al., 2008 |
(+) Indicates killer activity detected.
Identified from a culture isolate.
Identified from 16S rDNA clone library.
Identified by culture and non-culture dependent approaches.
The successful screening of the aguaniel and pulque for the isolation of diverse Leuconostoc and Lactobacillus species showing some in vitro and in vivo probiotic properties have been the subject of several reports (Table 4). These properties include:
Table 4.
Probiotic assessment of LAB isolated from aguamiel and pulque.
| Source and identity of studied LAB | Resistance to in vitro gastrointestinal exposition conditions | Other relevant in vitro or in vivo activity | References |
|---|---|---|---|
| Lactobacillus brevis isolated from pulque | This isolate strain showed 60% relative survival after acid exposition (pH 1.5), and 50–55% relative survival to simulated gastric acid exposition (pH 2.0). Bile tolerance to 0.3% taourocholic acid < 80%. Incubation conditions assayed: 4 h, 37° | Resistance to cefepime antibiotic, higher activity of bile salt hydrolase in MRS supplemented with 0.5% of taourocholic acid (671.72 U/mg protein) | González-Vázquez et al., 2015 |
| Leuconostoc mesenteroides subsp. mesenteroides isolated from aguamiel (four strains) | Isolates showed < 50% survival to acid exposition (pH 2, 3 h, 37°C). Bile tolerance to 0.5% oxgall (4 h, 37°C) | All strains showed resistance to dicloxacillin, pefloxacin, trimethoprim, ceftazidime antibiotics. In vitro antimicrobial activity of cell-free supernatants against Escherichia coli, Salmonella enterica and Listeria monocytogenes. Bacterial adherence to mice intestinal mucosa | Castro-Rodríguez et al., 2015 |
| Lactobacillus plantarum, L. paracasei subsp. paracasei, L. brevis, L. composti, L. sanfranciscensis isolated from pulque (14 isolates) | Two assayed strains showed >80% survival to lysozyme exposition. Three assayed strains showed > 80% survival to both acid pH (2.5) and 0.3% bile salts exposition. Exposition conditions assayed: 3 h, 37°C | Low binding capacity to HT-29 cells (~0.3%, best result) and to HT-29-MTX cells (10.78%, best result). In both assays, the binding capacity of isolated LAB was higher than control strain (L. casei BL23). Isolate identified as L. sanfranciscensis improve mice health by reduction of weight loss, significant decreases in gut permeability and anti-inflammatory effect by blocking the secretion of cytokines | Torres-Maravilla et al., 2016 |
| Lactobacillus brevis and L. collinoides isolated from aguamiel (14 isolates) | Resistant to an in vitro model simulating gastrointestinal conditions | Capable of dissociating conjugated bile salts by the presence of diverse bile salt hydrolases. Some isolates were resistant to dicloxacillin, pefloxacin and ceftazidime antibiotics. The isolated strain of L. brevis Lb9H showed in vivo protective effect of liver damage associated with the prevention of ALTa activity and preventing the intoxication by LPS+D-GalNb, indicator of lipid peroxidation | Reyes-Naya et al., 2016 |
| L. mesentreoides strain P45 isolated from pulque | Resistance to lysozyme exposition 70% (2 h, 37°C). 100% resistance to 0.3% and 1% bile salts exposition (4 h, 37°C). ~75% resistance to acid exposition (pH 2.5, 5 h, 37°C). This strain showed remarkable resistance to combined acid (pH 2.5) and bile salt (0.3%) exposition for 24 h, 37°C | In vitro antimicrobial activity against enteropathogenic E. coli, S. enterica serovar Typimurium, S. enterica serovar Typhi and L. monocytogenes in cell-to-cell assays (LAB-pathogen), cell-free supernatants assays and EPS-producing cell-to-cell assays (LAB-pathogen). In vivo assays showed that administration of strain P45 is associated with an important decrement in S. enterica serovar Typhimurium infection in liver and spleen in BALB/c female and male mice | Giles-Gómez et al., 2016 |
Serum alanine transferase.
Lipopolysaccharide + D-Galactosamine.
Resistance to antimicrobial barriers in the gastrointestinal tract such as lysozyme dilution by saliva, acid pH, gastric solution, and bile salt (Castro-Rodríguez et al., 2015; González-Vázquez et al., 2015; Giles-Gómez et al., 2016; Reyes-Naya et al., 2016; Torres-Maravilla et al., 2016).
Antimicrobial activity against pathogenic bacteria such as enteropathogenic Escherichia coli, Salmonella enterica serovar Typhimurium, S. enterica serovar Typhi and Listeria monocytogenes (Castro-Rodríguez et al., 2015; González-Vázquez et al., 2015; Giles-Gómez et al., 2016; Torres-Maravilla et al., 2016).
In vivo adherence to mice intestinal mucosa (Castro-Rodríguez et al., 2015).
In vivo anti-inflammatory activity in a mouse model (Torres-Maravilla et al., 2016).
In vivo anticholesterolemic affect (Reyes-Naya et al., 2016).
In vivo anti-infective effect against S. enterica serovar Typhymurium (Giles-Gómez et al., 2016).
This scientific evidence of LAB responsibility for health-promoting effects associated with pulque consumption makes these bacteria relevant probiotic candidates for the development of non-dairy based functional products.
Functional properties of EPS produced by LAB detected in aguamiel and pulque
Some EPS produced by LAB isolated from aguamiel and pulque have been purified and characterized. Results include the identification of dextran with a linear backbone linked in α1 → 6 D-Glcp linkages with branching in α1 → 3 D-Glcp produced by a cell-associated glycosyltransferase (GTF) from L. mesenteroides isolated from pulque collected from the Apan region, in the state of Hidalgo (Chellapandian et al., 1998). In the same context, two EPS LAB identified as L. kimchii were isolated from pulque produced in Huitzilac, in the state of Morelos. One of the strains (EPSA) produced dextran with a linear backbone joined by α1 → 6 D-Glcp with α1 → 2 and α1 → 3 branching linkages through enzymes found in the soluble and the cell-associated fractions. The second strain (EPSB) produced a polymer mixture including a levan composed by linear chains containing β2 → 6 linked β-D-fructofuranosyl moieties and β2 → 1 branches (79%), as well as a dextran Type I polymer (21%) (Torres-Rodríguez et al., 2014).
EPS and hetero-oligosaccharides produced by diverse LAB species, including those found in pulque, have gained attention because of their use as food additives and potential natural functional ingredients. Their main applications include their use as prebiotic agents as well as soluble fiber (Patel et al., 2011; Harutoshi, 2013) such as those produced by Lactobacillus reuteri, L. rhamnosus, L. acidophilus, and Bifidobacterium bifidum (Helal et al., 2015). EPS produced by LAB with potential probiotic properties have been proposed to play a positive effect in the intestinal adhesion (García-Ruiz et al., 2014). In vitro antimicrobial assays with EPS-producing L. mesenteroides strain P45 isolated from pulque against EPEC E. coli, S. enterica serovar Typhimurium, S. enterica serovar Typhi, and L. monocytogenes showed an improved in vitro antimicrobial activity in EPS-producing cell-to-cell assays (Giles-Gómez et al., 2016). These results are preliminary, as the detailed mechanisms involved both in vivo and in-vitro potential functional properties of EPS produced by LAB, particularly those species assayed for potential probiotic activities remain to be determined.
Pulque industrialization and major technological challenges
Science and technology of pulque
A simple look at research figures illustrates the lack of interest in pulque by the scientific community: A PubMed search under “beer” results in today in 17,929 hits while only 30 references come out under “pulque” most of them published in the twenty-first century. However, 8 of them were released in the last 2 years (2014 and 2015) as evidence of a renew interest.
It is worthwhile looking at this extremely low figure in more detail, as the earliest scientific publication dealing with the process, dates back to 1957 when Alfredo Sanchez Marroquin (Sánchez-Marroquín et al., 1957), first tried to industrialize pulque starting from the basic/minimum microbiological requirements to transform aguamiel into pulque. We, of course, acknowledge the initial efforts of Dr. Leopoldo Río de la Loza to elucidate the microbiology of pulque in 1864. He reported in the Boletin de la Sociedad de Geografía y Estadística, the isolation of Termobacterium mobile by Paul Lindner in 1924 (Weir, 2016), among others. Pulque's microbiology, the isolation of strains, and more recently, its individual probiotic characterization, is probably the main research trend (Torres-Rodríguez et al., 2014; Castro-Rodríguez et al., 2015; González-Vázquez et al., 2015; Giles-Gómez et al., 2016; Torres-Maravilla et al., 2016). An additional research subject deals with the effect of pulque in the Mexican diet. The first reference given by PubMed is a document from 1897 in which Francisco Martínez Baca, a famous physician from the state of Puebla described the successful treatment with pulque of penitentiary inmates suffering scurvy (published in the Journal of the American Public Health Association) (Ramírez Rancaño, 2000). It was not until 1933 that vitamin C was finally discovered (Carpenter, 2012).
However, no references deal with pulque production technology, scaling up of the process, neither the definition of the main microbiota required to reproduce the beverage, as consumers know it. These concerns remain as technological challenges since last century when Sanchez Marroquin defined the four physiological processes involved in pulque production (Sánchez-Marroquín et al., 1957). Nevertheless, reducing the microbiota to three or four microorganisms would blindly eliminate possible bacteria contributing as probiotics to the claimed beneficial health effects, particularly to treat gastrointestinal problems and diarrhea. The simple decision between S. cerevisiae or Z. mobilis as the alcohol producer is not that evident. S. cerevisiae reaches higher ethanol concentrations without inhibition, while Z. mobilis, a faster ethanol producer, also contains two levansucrases, responsible for levan synthesis, part of the soluble fiber in which pulque is particularly rich (Lau et al., 2010; Xiong He et al., 2014; Weir, 2016). Up to now, pulque remains as a very heterogeneous beverage regarding its common final organoleptic properties (alcohol-acid taste and viscosity): while many drinkers prefer the fresh product, others prefer pulque after more than 24 h of fermentation combined with fruit juice (curados). Nevertheless, pulque does not stand large storage times without developing off flavors, and pasteurization not only affects flavor but also destroys one of its main properties: the microbiota.
It is probably to this aspect that the largest (but still minor) efforts in research have been devoted. The presence of prebiotic fructooligosaccharides from agave inulin present in aguamiel, as well as the soluble inulin-like agavin, levan and dextran polysaccharides have been described and characterized (Chellapandian et al., 1998; Ortiz-Basurto et al., 2008; Torres-Rodríguez et al., 2014). Some of this prebiotics have been evaluated both in vitro and in vivo, and we suggest that the beneficial effects observed among lactating mothers and their babies (Argote-Espinosa et al., 1992; Backstrand et al., 2001, 2004) is mainly due to its pre- and probiotic content. Unfortunately, most research is now devoted to the isolation and production of probiotic bacteria as alternative beverages, isolated from pulque, but out of the scope of the beverage. These efforts are similar to those carried out last century by Paul Lindner himself. He was convinced that Pseudomonas lindneri (that he had previously defined as Thermobacterium mobile) was responsible for the beneficial effects of pulque in the treatment of intestinal disorders and produced in Berlin from this single bacteria a “functional” fermented beverage (Gonçalves de Lima, 1956).
Challenges associated with pulque production
Probably the main challenge associated with the industrialization of pulque is related to the natural substrate availability and the need for the introduction of a stabilization processes of the fermented product. Aguamiel differs from almost all other fermented beverages such as wine, beer or tepache (pineapple wine), in that agave, the raw material, takes 7 years to reach maturity. Furthermore, when ready for production, aguamiel has to be collected from the plant on a daily basis, and not produced by a single extraction, as it is usually the case for fermented beverages. Each agave plant is visited daily during several months and the accumulated aguamiel extracted, a labor-intensive activity, which also induce fermentation in the plant itself where aguamiel accumulates during the day. Therefore, the fermentation is already taking place when the substrate is collected. In contrast, the fermentation that leads to tequila or mezcal, also produced from agave sugars, does not require this process as sugars are extracted directly from the mature plant (Agave tequilana) in a single operation after the agave pine is cooked and mashed.
Several successful efforts for industrialization for the production of bottled/canned fermented pulque have been performed mainly by producers in the States of Puebla, Tlaxcala and Hidalgo (Ramírez et al., 2004; Jaurez Rosas, 2015). The producers include companies as Tecnología e Innovación en Pulque Industrial S.A. de C.V., comprising more than 300 pulque producers in Puebla state, Torre Grande in Hidalgo and Procesadora de Pulque S.A. de C.V and Pulque Hacienda 1881 in Tlaxcala. Both companies export canned pulque to Europe, Central America, and the United States, the latter being the largest market for canned pulque (mainly the cities of Los Angeles and Chicago where are the biggest settlement of Mexican immigrants) (Jaurez Rosas, 2015). However, the industrialization of pulque introduced fundamental changes in the public perception of traditional producers and consumers resulting in a product that the majority of traditional consumers never tasted before. Efforts to stabilize the fermented beverage by pasteurizing and/or filtrate pulque or by the addition of preservatives, antioxidants, colorants or texturizing agents will certainly improve stability and shelf life but could reduce the pre- and probiotic content of the fermented beverage (Ramírez et al., 2004; Escalante et al., 2012).
However, there is an increasing preference for local products and local markets (Jaurez Rosas, 2015). We believe that the main scientific and technological investment should come from the demonstration of the main nutritional, health-promoting and organoleptic attributes of pulque and its microbiota, introducing specific modifications in the traditional production tinacales that bring assurance to the consumer that pulque is produced hygienically, conserving its local characteristics and its regular strains, but safe to the consumer.
Functional genomics of pulque and relevant microorganisms involved in the fermentation process
Application of a culture-independent approach such as 16S rDNA clone library to the study of bacterial diversity present in aguamiel and pulque allowed to determine a remarkable LAB diversity, suggesting an essential role of these microorganisms in the fermentation process (Escalante et al., 2004, 2008). Emerging research on the microbiology of pulque focuses on the isolation and in vitro as in vivo assessment of probiotic LAB with promising capabilities (Castro-Rodríguez et al., 2015; González-Vázquez et al., 2015; Giles-Gómez et al., 2016; Reyes-Naya et al., 2016; Torres-Maravilla et al., 2016; Table 4).
Functional genomics from available LAB genome information has provided new insights regarding the evolution of LAB, their metabolic profile and the interactions with other microorganisms and the environment, allowing to understand the role of these microorganisms in traditional or industrial food fermentations and their interactions with the human hosts (Douillard and de Vos, 2014). Genome sequencing of relevant LAB isolated from pulque, such as those recently identified with potential probiotic properties promises to provide valuable information on the genetic traits involved in the probiotic activity.
Complete genome analysis of potential probiotic L. mesenteroides strain P45 by Riveros-McKay et al. (2014), allowed the identification of diverse genes probably involve in the antimicrobial activity of this LAB such as those coding for diverse peptidoglycan hydrolases and a prebacteriocin (Giles-Gómez et al., 2016). This information provides new insights to focus further efforts on the characterization of the potential probiotic of this LAB from pulque. However, the next step in the study of pulque microbiology relies on the application of metagenomic approaches to study the entire microbial composition (including both bacteria and yeasts) in combination with other high-throughput omic methodologies such as transcriptomics, metabolomics or proteomics. These approaches applied to other regional traditional fermented foods and beverages (e.g., Korean kimchi Jung et al., 2011), could provide valuable insights into the complex microbial community involved in the fermentation process.
Concluding remarks
All through Mexican history, from pre-hispanic times to our days, pulque has been a key reference regarding culture, tradition, and cuisine. Once the center of the cosmological vision of our ancestors, later a source of wealth through agro-industrial exploitation, abandoned and despised -described as a nutrient of underdevelopment and ignorance after the Revolution Civil War, and now the subject of wonder and scientific research. Pulque is now the center of research in many laboratories, not only due to its nutritional properties but also to the extremely complex microbial diversity responsible for its fermentation, a process that has resisted industrialization. No doubt, pulque is an essential element for the UNESCO decision in 2010 to include the traditional Mexican cuisine in the List of the Intangible Cultural Heritage of Humanity.
Author contributions
DL and JV collected the video and photographic material included in this contribution and prepared the information corresponding to the traditional process of pulque fermentation. AE, MG, FB, and AL wrote the manuscript and designed the graphic material. All the authors reviewed and approved the final version of the manuscript.
Funding
This contribution was supported by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT), UNAM project IN207914.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Mr. José Pérez Cerón from Tlamaco, Atitlaquia Hidalgo for the facilities for recording the castration process. To Mr. Salvador Cueto, from Huitzilac, Morelos for their kindly support for some of the images included in this contribution.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.01026
Castration process of a mature maguey for aguamiel production 1. (Video in mp4 format, 2:27 min). Pulque producer or tlachiquero perform castration process. Once the plant has been selected the tlachiquero prepares the maguey by cutting off the central leaves of the plant surrounding the floral bud with a sharpened knife. With the floral peduncle exposed (“opening the door”), the tlachiquero cut off this part of the plant with a knife.
Castration process of a mature maguey for aguamiel production 2. (Video in mp4 format, 2:03 min). The remaining floral bud is destroyed to avoid the possible development of the embryonic floral peduncle. For this operation, the tlachiquero uses a pointed and sharpen metallic instrument (a jimmy bar) to make a pit in the residual floral bud (0:00–0:43 min). Finally, the tlachiquero uses a scraping tool to make the final shape of the cavity (cajete) and covers the pit with a maguey leaf (0:43–2:03 min).
References
- Alfaro Rojas G., Legaria Solano J. P., Rodríguez Pérez J. E. (2007). Diversidad genética en poblaciones de agaves pulqueros (Agave spp.) del noroeste del Estado de México. Rev. Fitotec. Mex. 30, 1–12. [Google Scholar]
- Anawalt P. R. (1998). Los conejos y la embriaguez. Arqueol. Mex. 9, 66–73. [Google Scholar]
- Anderson R. K., Calvo J., Serrano G., Payne G. C. (1946). A study of the nutritional status and food habits of Otomi indians in the Mezquital Valley of Mexico. Am. J. Public Health Nations Health 36, 883–903. 10.2105/AJPH.36.8.883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argote-Espinosa R. M., Flores-Huerta S., Hernández-Montes H., Villalpando-Hernández S. (1992). Plasma clearance of ethanol and its excretion in the milk of rural women who consume pulque. Rev. Investig. Clínica Organo Hosp. Enfermedades Nutr. 44, 31–36. [PubMed] [Google Scholar]
- Backstrand J. R., Allen L. H., Black A. K., de Mata M., Pelto G. H. (2002). Diet and iron status of nonpregnant women in rural Central Mexico. Am. J. Clin. Nutr. 76, 156–164. [DOI] [PubMed] [Google Scholar]
- Backstrand J. R., Allen L. H., Martinez E., Pelto G. H. (2001). Maternal consumption of pulque, a traditional central Mexican alcoholic beverage: relationships to infant growth and development. Public Health Nutr. 4, 883–891. 10.1079/PHN2001130 [DOI] [PubMed] [Google Scholar]
- Backstrand J. R., Goodman A. H., Allen L. H., Pelto G. H. (2004). Pulque intake during pregnancy and lactation in rural Mexico: alcohol and child growth from 1 to 57 months. Eur. J. Clin. Nutr. 58, 1626–1634. 10.1038/sj.ejcn.1602019 [DOI] [PubMed] [Google Scholar]
- Carpenter K. J. (2012). The discovery of vitamin C. Ann. Nutr. Metab. 61, 259–264. 10.1159/000343121 [DOI] [PubMed] [Google Scholar]
- Carr F. J., Chill D., Maida N. (2002). The lactic acid bacteria: a literature survey. Crit. Rev. Microbiol. 28, 281–370. 10.1080/1040-840291046759 [DOI] [PubMed] [Google Scholar]
- Castro-Rodríguez D., Hernández-Sánchez H., Yáñez Fernández J. (2015). Probiotic properties of Leuconostoc mesenteroides isolated from aguamiel of Agave salmiana. Probiotics Antimicrob. Proteins 7, 107–117. 10.1007/s12602-015-9187-5 [DOI] [PubMed] [Google Scholar]
- Castro-Zavala A., Juárez-Flores B. I., Pinos-Rodríguez J. M., Delgado-Portales R. E., Aguirre-Rivera J. R., Alcocer-Gouyonnet F. (2015). Prebiotic effects of Agave salmiana fructans in Lactobacillus acidophilus and Bifidobacterium lactis cultures. Nat. Prod. Commun. 10, 1985–1988. [PubMed] [Google Scholar]
- Chellapandian M., Larios C., Sanchez-Gonzalez M., Lopez-Munguia A. (1998). Production and properties of a dextransucrase from Leuconostoc mesenteroides IBT-PQ isolated from “pulque,” a traditional Aztec alcoholic beverage. J. Ind. Microbiol. Biotechnol. 21, 51–56. 10.1038/sj.jim.2900560 [DOI] [Google Scholar]
- Correa-Ascencio M., Robertson I. G., Cabrera-Cortes O., Cabrera-Castro R., Evershed R. P. (2014). Pulque production from fermented agave sap as a dietary supplement in Prehispanic Mesoamerica. Proc. Natl. Acad. Sci. U.S.A. 111, 14223–14228. 10.1073/pnas.1408339111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist R. (1939). The pulque industry 1939. Econ. Geogr. 15, 189–194. 10.2307/141429 [DOI] [Google Scholar]
- Douillard F. P., de Vos W. M. (2014). Functional genomics of lactic acid bacteria: from food to health. Microb. Cell. Fact. 13:S8. 10.1186/1475-2859-13-S1-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante A., Giles-Gómez M., Esquivel Flores G., Matus Acuña V., Moreno-Terrazas R., López-Munguía A., et al. (2012). Pulque fermentation, in Handbook of Plant-Based Fermented Food and Beverage Technology, ed Hui Y. H. (Boca Raton, FL: CRC Press; ), 691–706. [Google Scholar]
- Escalante A., Giles-Gómez M., Hernandez G., Cordova-Aguilar M., Lopez-Munguia A., Gosset G., et al. (2008). Analysis of bacterial community during the fermentation of pulque, a traditional Mexican alcoholic beverage, using a polyphasic approach. Int. J. Food Microbiol. 124, 126–134. 10.1016/j.ijfoodmicro.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Escalante A., Rodriguez M. E., Martinez A., López-Munguía A., Bolivar F., Gosset G. (2004). Characterization of bacterial diversity in Pulque, a traditional Mexican alcoholic fermented beverage, as determined by 16S rDNA analysis. FEMS Microbiol. Lett. 235, 273–279. 10.1111/j.1574-6968.2004.tb09599.x [DOI] [PubMed] [Google Scholar]
- Estrada-Godina A. R., Cruz-Guerrero A. E., Lappe P., Ulloa M., García-Garibay M., Gómez-Ruiz L. (2001). Isolation and identification of killer yeasts from Agave sap (aguamiel) and pulque. World J. Microbiol. Biotechnol. 17, 557–560. 10.1023/A:1012210106203 [DOI] [Google Scholar]
- García-Aguirre M., Sáenz-Álvaro V. A., Rodríguez-Soto M. A., Vicente-Magueyal F. J., Botello-Álvarez E., Jimenez-Islas H., et al. (2009). Strategy for biotechnological process design applied to the enzymatic hydrolysis of agave fructo-oligosaccharides to obtain fructose-rich syrups. J. Agric. Food Chem. 57, 10205–10210. 10.1021/jf902855q [DOI] [PubMed] [Google Scholar]
- García-Garibay M., López-Munguía A. (1993). Bebidas alcohólicas no destiladas, in Biotecnología Alimentaria, eds García-Garibay M., Quintero Ramírez R., López-Munguía A. (México: LIMUSA; ), 263–311. [Google Scholar]
- García-Ruiz A., González de Llano D., Esteban-Fernández A., Requena T., Bartolomé B., Moreno-Arribas M. V. (2014). Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiol. 44, 220–225. 10.1016/j.fm.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Giles-Gómez M., Sandoval García J. G., Matus V., Campos Quintana I., Bolívar F., Escalante A. (2016). In vitro and in vivo probiotic assessment of Leuconostoc mesenteroides P45 isolated from pulque, a Mexican traditional alcoholic beverage. SpringerPlus 5:708 10.1186/s40064-016-2370-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves de Lima O. (1956). El maguey y el Pulque en los Códices Mexicanos. México DF: Fondo de Cultura Económica. [Google Scholar]
- González-Vázquez R., Azaola-Espinosa A., Mayorga-Reyes L., Reyes-Nava L. A., Shah N. P., Rivera-Espinoza Y. (2015). Isolation, identification and partial characterization of a Lactobacillus casei strain with bile salt hydrolase activity from pulque. Probiotics Antimicrob. Proteins 7, 242–248. 10.1007/s12602-015-9202-x [DOI] [PubMed] [Google Scholar]
- Good-Avila S. V., Souza V., Gaut B. S., Eguiarte L. E. (2006). Timing and rate of speciation in Agave (Agavaceae). Proc. Natl. Acad. Sci. U.S.A. 103, 9124–9129. 10.1073/pnas.0603312103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harutoshi T. (2013). Exopolysaccharides of lactic acid bacteria for food and colon health applications, in Lactic Acid Bacteria - R & D for Food, Health and Livestock Purposes, ed Kongo J. M. (Rijeka: InTech; ), 515–538. [Google Scholar]
- Helal M., Hussein M.-D., Osman M., Shalaby A. S., Ghaly M. (2015). Production and prebiotic activity of exopolysaccharides derived from some probiotics. Egypt. Pharm. J. 14, 1 10.4103/1687-4315.154687 [DOI] [Google Scholar]
- INEGI (2016). Instituto Nacional de Estadística y Geografía. INEGI. Available online at: http://www.inegi.org.mx/default.aspx [Accessed May 22, 2016].
- Instituto Nacional de Salud Pública (2011). Encuesta Nacional de Adicciones. Available online at: http://encuestas.insp.mx/ena/ena2011.html#.V0Eiu77-vOU [Accessed May 22, 2016].
- Jácome A. G. (2003). Cultura y Agricultura: Transformaciones en el Agro Mexicano. México DF: Universidad Iberoamericana. [Google Scholar]
- Jaurez Rosas V. B. (2015). Proyecto de Inversión para la Instalación y Comercialización de una Planta Envasadora de Pulque. Dissertation/BSc thesis, México DF., Universidad Nacional Autónoma de México. [Google Scholar]
- Jennings J., Antrobus K. L., Atencio S. J., Glavich E., Johnson R., Loffler G., et al. (2005). “Drinking beer in a blissful mood”: alcohol production, operational chains, and feasting in the Ancient World. Curr. Anthropol. 46, 275–303. 10.1086/427119 [DOI] [Google Scholar]
- Jung J. Y., Lee S. H., Kim J. M., Park M. S., Bae J.-W., Hahn Y., et al. (2011). Metagenomic analysis of kimchi, a traditional Korean fermented food. Appl. Environ. Microbiol. 77, 2264–2274. 10.1128/AEM.02157-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Oliveras P., Moreno-Terrazas R., Arrizón-Gaviño J., Herrera-Suárez T., García-Mendoza A., Gschaedler-Mathis A. (2008). Yeasts associated with the production of Mexican alcoholic nondistilled and distilled Agave beverages. FEMS Yeast Res. 8, 1037–1052. 10.1111/j.1567-1364.2008.00430.x [DOI] [PubMed] [Google Scholar]
- Lau M. W., Gunawan C., Balan V., Dale B. E. (2010). Research comparing the fermentation performance of Escherichia coli KO11, Saccharomyces cerevisiae 424A (LNH-ST) and Zymomonas mobilis AX101 for cellulosic ethanol production. Biotechnol. Biofuels 3:11. 10.1186/1754-6834-3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo Monterrubio A. (2007). El maguey y el pulque en Méxcio, in Las Haciendas Pulqueras de México (México: UNAM, Coordinación de Estudios de Posgrado, Programa de Posgrado en Arquitectura; ), 41–63. [Google Scholar]
- Morales de León J., Bourges H., Camacho M. E. (2005). Aminoacid composition of some Mexican foods. Arch. Latinoaméricanos Nutr. 55, 172–186. [PubMed] [Google Scholar]
- Mora-López J. L., Reyes-Agüero J. A., Flores-Flores J. L., Peña-Valdivia C. B., Aguirre-Rivera J. R. (2011). Variación morfológica y humanización de la sección Salmianae del género Agave. Agrociencia 45, 465–477. [Google Scholar]
- Narro-Robles J., Gutiérrez-Avila M. C. (1997). Correlación ecológica entre consumo de bebidas alcohólicas y mortalidad por cirrosis hepática en México. Salud Públ. Méx. 39, 217–220. 10.1590/S0036-36341997000300007 [DOI] [PubMed] [Google Scholar]
- Ortiz-Basurto R. I., Pourcelly G., Doco T., Williams P., Dornier M., Belleville M.-P. (2008). Analysis of the main components of the aguamiel produced by the maguey-pulquero (Agave mapisaga) throughout the harvest period. J. Agric. Food Chem. 56, 3682–3687. 10.1021/jf072767h [DOI] [PubMed] [Google Scholar]
- Parsons J. R., Darling J. A. (2000). Maguey (Agave spp.) utilization in Mesoamerican civilization: a case for Precolumbian “pastoralism.” Bol. Soc. Bot. Méx. 66, 81–91. [Google Scholar]
- Patel S., Majumder A., Goyal A. (2011). Potentials of exopolysaccharides from lactic acid bacteria. Indian J. Microbiol. 52, 3–12. 10.1007/s12088-011-0148-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez E. (2002). Historia del sabio señor Quetzalcóatl. Arqueol. Mex. 6, 50–53. [Google Scholar]
- Ramírez J. F., Sánchez-Marroquín A., Álvarez M. M., Valyasevi R. (2004). Industrialization of Mexican pulque, in Industrialization of Indigenous Fermented Foods, ed Steinkraus K. H. (New York, NY: Marcel Decker; ), 547–585. [Google Scholar]
- Ramírez Rancaño M. (2000). El Rey del Pulque: Ignacio Torres Adalid y la Industria Pulquera. México: Plaza y Valdes. UNAM, Instituto de Investigaciones Sociales. [Google Scholar]
- Ramírez Rodríguez R. (2004). El Maguey y el Pulque: Memoria y Tradición Convertidas en Historia, 1884-1993. Dissertation/BSc thesis, Puebla(PUE), Benemérita Universidad Autónoma de Puebla. [Google Scholar]
- Raspor P., Goranovič D. (2008). Biotechnological applications of acetic acid bacteria. Crit. Rev. Biotechnol. 28, 101–124. 10.1080/07388550802046749 [DOI] [PubMed] [Google Scholar]
- Reyes-Naya L., Garduño-Siciliano L., Santos E., Hernández-Sánchez H. A., Arauz J., Muriel P., et al. (2016). Use of bile acids as a selection strategy for lactobacillus strains with probiotic potential. J. Food Nutr. Disord. 5:1 10.4172/2324-9323.1000187 [DOI] [Google Scholar]
- Riveros-McKay F., Campos I., Giles-Gomez M., Bolivar F., Escalante A. (2014). Draft genome sequence of Leuconostoc mesenteroides P45 isolated from pulque, a traditional Mexican alcoholic fermented beverage. Genome Announc. 2, e01130–14–e01130–14. 10.1128/genomeA.01130-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahagún B. D. (ed.). (1999). Historia General de las Cosas de la Nueva España, 10th Edn. México DF: Porrúa. [Google Scholar]
- Sánchez-Marroquín A., Hope P. H. (1953). Agave juice, fermentation and chemical composition studies of some species. J. Agric. Food Chem. 1, 246–249. 10.1021/jf60003a007 [DOI] [Google Scholar]
- Sánchez-Marroquín A., Terán J., Piso J. (1957). Estudios sobre la microbiología del pulqe. -XVIII.- Datos químicos de la fermentación de aguamiel con cultivos puros. Rev. Soc. Quím. México 1, 167–174. [Google Scholar]
- Santos-Zea L., Leal-Díaz A. M., Jacobo-Velázquez D. A., Rodríguez-Rodríguez J., García-Lara S., Gutiérrez-Uribe J. A. (2016). Characterization of concentrated agave saps and storage effects on browning, antioxidant capacity and amino acid content. J. Food Compos. Anal. 45, 113–120. 10.1016/j.jfca.2015.10.005 [DOI] [Google Scholar]
- Secretaría de Economía (1972a). Aguamiel. Normas Mex. Vigen. Available online at: http://www.economia-nmx.gob.mx/normasmx/detallenorma.nmx?clave=NMX-V-022-1972 [Accessed May 17, 2016].
- Secretaría de Economía (1972b). Pulque Manejado a Granel. Normas Mex. Vigen. Available online at: http://www.economia-nmx.gob.mx/normasmx/detallenorma.nmx?clave=NMX-V-037-1972 [Accessed May 17, 2016].
- Silos-Espino G., González-Cortés N., Carrillo-López A., Guevaralara F., Valverde-González M. E., Paredes-López O. (2007). Chemical composition and in vitro propagation of Agave salmiana “Gentry.” J. Hortic. Sci. Biotechnol. 82, 355–359. 10.1080/14620316.2007.11512242 [DOI] [Google Scholar]
- Soccol C. R., De Dea J., Tiemi C., Rigan M., Porto de Souza L., Soccol T. (2012). Probiotic nondairy beverages, in Handbook of Plant-Based Fermented Food and Beverage Technology, ed Hui Y. H. (Boca Raton, FL: CRC Press; ), 707–728. [Google Scholar]
- The Plant List (2010). Version 1 Plant List Work. List Plant Species. Available online at: http://www.theplantlist.org/cite/ [Accessed April 3, 2016].
- Torres-Maravilla E., Lenoir M., Mayorga-Reyes L., Allain T., Sokol H., Langella P., et al. (2016). Identification of novel anti-inflammatory probiotic strains isolated from pulque. Appl. Microbiol. Biotechnol. 100, 385–396. 10.1007/s00253-015-7049-4 [DOI] [PubMed] [Google Scholar]
- Torres-Rodríguez I., Rodríguez-Alegría M. E., Miranda-Molina A., Giles-Gómez M., Morales R. C., López-Munguía A., et al. (2014). Screening and characterization of extracellular polysaccharides produced by Leuconostoc kimchii isolated from traditional fermented pulque beverage. SpringerPlus 3:583. 10.1186/2193-1801-3-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar L. R., Olivos M., Gutierrez M. E. (2008). Pulque, an alcoholic drink from rural Mexico, contains phytase. Its in vitro effects on corn tortilla. Plant Foods Hum. Nutr. 63, 189–194. 10.1007/s11130-008-0089-5 [DOI] [PubMed] [Google Scholar]
- Tripathi M. K., Giri S. K. (2014). Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 9, 225–241. 10.1016/j.jff.2014.04.030 [DOI] [Google Scholar]
- Valadez-Blanco R., Bravo-Villa G., Santos-Sánchez N. F., Velasco-Almendarez S. I., Montville T. J. (2012). The artisanal production of pulque, a traditional beverage of the Mexican Highlands. Probiotics Antimicrob. Proteins 4, 140–144. 10.1007/s12602-012-9096-9 [DOI] [PubMed] [Google Scholar]
- Velázquez-Martínez J., González-Cervantes R., Hernández-Gallegos M., Mendiola R., Aparicio A., Ocampo M. (2014). Prebiotic potential of Agave angustifolia Haw fructans with different degrees of polymerization. Molecules 19, 12660–12675. 10.3390/molecules190812660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir P. M. (2016). The ecology of Zymomonas: a review. Folia Microbiol. (Praha). [Epub ahead of print]. 10.1007/s12223-016-0447-x [DOI] [PubMed] [Google Scholar]
- Wilson I., Pineda A. (1963). Pineda's report on the beverages of New Spain. Ariz. West 5, 79–90. [Google Scholar]
- Xiong He M., Wu B., Ruan Yong Z., Rong Tan F., Li Wang J., Xia Shui Z., et al. (2014). Zymomonas mobilis: a novel platform for future biorefineries. Biotechnol. Biofuels 7:101. 10.1186/1754-6834-7-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Castration process of a mature maguey for aguamiel production 1. (Video in mp4 format, 2:27 min). Pulque producer or tlachiquero perform castration process. Once the plant has been selected the tlachiquero prepares the maguey by cutting off the central leaves of the plant surrounding the floral bud with a sharpened knife. With the floral peduncle exposed (“opening the door”), the tlachiquero cut off this part of the plant with a knife.
Castration process of a mature maguey for aguamiel production 2. (Video in mp4 format, 2:03 min). The remaining floral bud is destroyed to avoid the possible development of the embryonic floral peduncle. For this operation, the tlachiquero uses a pointed and sharpen metallic instrument (a jimmy bar) to make a pit in the residual floral bud (0:00–0:43 min). Finally, the tlachiquero uses a scraping tool to make the final shape of the cavity (cajete) and covers the pit with a maguey leaf (0:43–2:03 min).