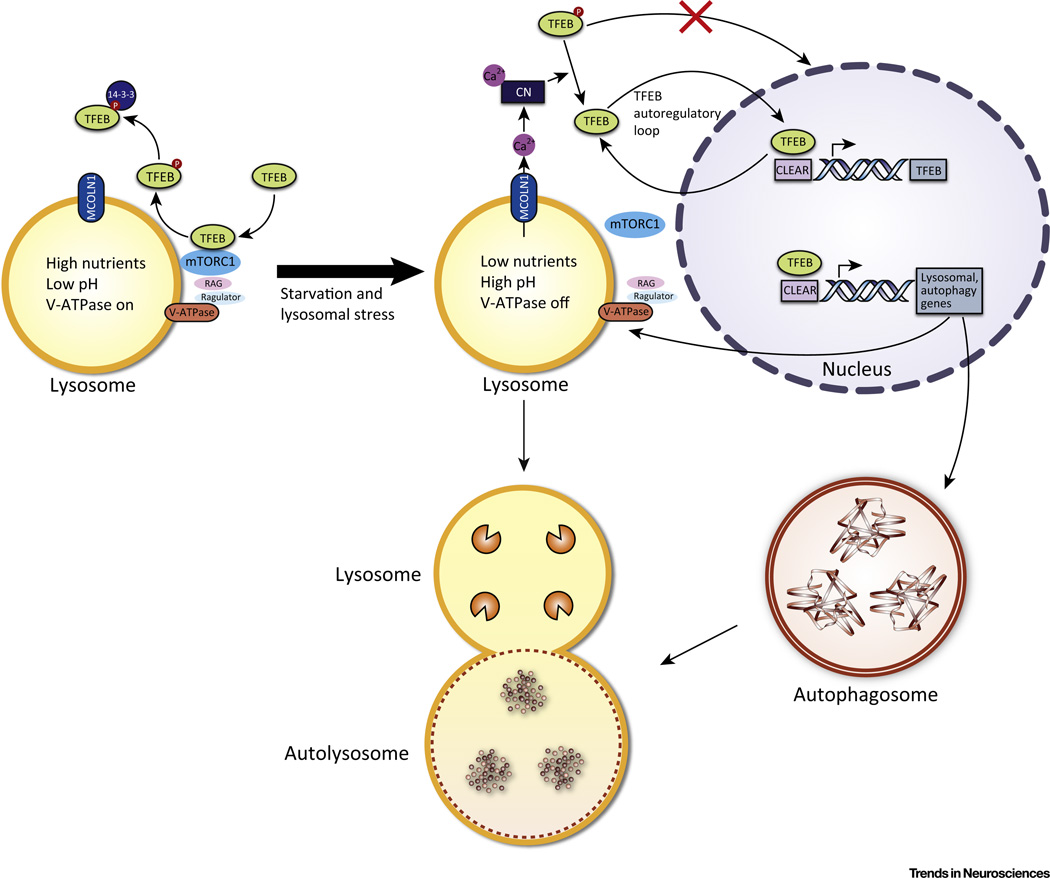
Figure 3. Regulation and Activity of TFEB.
In conditions of normal nutrient availability, no lysosomal stress, and no V-ATPase inhibition, the V-ATPase, Ragulator, and Rag GTPases form an active complex that binds to mTORC1 at the lysosomal surface and activates it. mTORC1 then phosphorylates (P) TFEB which sequesters it in the cytoplasm. Phosphorylated TFEB is also bound by 14-3-3 protein in the cytoplasm. In conditions of starvation, lysosomal stress, or V-ATPase inhibition, the Rag GTPases are turned off, which releases mTORC1 from the lysosomal surface and inactivates it. Because mTORC1 can no longer phosphorylate TFEB, the unphosphorylated TFEB translocates to the nucleus to bind to the CLEAR sequence of its target genes, leading to the upregulation of autophagy and lysosomal genes. The TFEB gene also has numerous CLEAR sequences in its promoter, and thus TFEB upregulates its own expression in an autoregulatory loop. Another positive feedback loop deals with MCOLN1, a lysosomal calcium efflux channel that is a transcriptional target of TFEB. MCOLN1 creates a microdomain of high Ca2+ concentration near the lysosomal surface. The higher Ca2+ concentration then activates the phosphatase calcineurin (CN), which dephosphorylates TFEB, promoting its activation.