Abstract
The γ-secretase complex is composed of at least four components: presenilin (PS1 or PS2), nicastrin (NCT), anterior pharynx-defective 1 (Aph-1), and presenilin enhancer 2 (pen-2). In this study, using knockout cell lines, our data demonstrated that knockout of NCT, as well as knockout of Pen-2, completely blocked γ-secretase-catalyzed processing of CTFα and CTFβ, the C-terminal fragments of β-amyloid precursor protein (APP) produced by α-secretase and β-secretase cleavages, respectively. Interestingly, in Aph-1-knockout cells CTFα and CTFβ were still processed by γ-secretase, indicating Aph-1 is dispensable for APP processing. Furthermore, our results indicate that Aph-1 as well as NCT is not absolutely required for Notch processing, suggesting that NCT is differentially required for APP and Notch processing. In addition, our data revealed that components of the γ-secretase complex are also important for proteasome- and lysosome-dependent degradation of APP and that endogenous APP is mostly degraded by lysosome while exogenous APP is mainly degraded by proteasome.
Keywords: Alzheimer’s disease, APP, nicastrin, Aph-1, gamma-secretase
One of the hallmarks of Alzheimer's disease (AD) is the abnormal production and accumulation of β-amyloid peptide (Aβ) in the brain. According to the amyloid hypothesis, the ratio of the long Aβ species, Aβ42, versus the short Aβ40 (Aβ42/Aβ40) has been considered to play a critical role in AD (Hardy & Selkoe 2002). An increased Aβ42/Aβ40 ratio appears to correlate with early-onset familial AD cases caused by presenilin mutations (Kumar-Singh et al. 2006). Aβ is derived from the amyloid precursor protein (APP) by successive action of the β- and γ-secretases. APP can be processed via two pathways, the non-amyloidogenic pathway or the amyloidogenic pathway. In the non-amyloidogenic pathway, APP is first cleaved by α-secretase to release a soluble N-terminal ectodomain and a membrane anchored C-terminal fragment (CTFα); in the amyloidogenic pathway, APP is first cleaved by β-secretase to remove the N-terminal fragment and generate a membrane-anchored C-terminal fragment of APP (CTFβ). Both CTFα and CTFβ are then subsequently cleaved within the transmembrane domain by γ-secretase to produce a common APP intracellular domain (AICD) and lead to the generation of a p3 fragment from CTFα and the full-length Aβ from CTFβ (Xu 2009). Since the γ-secretase-catalyzed cleavage determines the C-termini of Aβ species and the ratio of Aβ42/Aβ40, dissecting the biological and biochemical nature of γ-secretase is important for understanding the mechanism of Aβ formation. Thus far at least four polypeptides have been identified as necessary components for γ-secretase activity (Dries & Yu 2008; Zhang et al. 2014). These four components are presenilins (PS1 or PS2), nicastrin (NCT), anterior pharynx-defective 1 (Aph-1), and presenilin enhancer 2 (Pen-2). Mutation of the two conserved aspartyl residues in PS1 and PS2 results in the loss of γ-secretase activity (Wolfe 1999), and affinity labeling experiments have demonstrate that γ-secretase inhibitors bind directly to PS1 (Esler et al. 2000; Li et al. 2000); therefore, the nine transmembrane protein presenilin (PS1 or PS2 isoforms) is thought to function as the catalytic subunit of γ-secretase (Wolfe 2002). The identification of a substrate-binding domain in NCT strongly suggests that NCT functions as the substrate receptor (Shah et al. 2005). Using siRNA technology, studies suggested that the seven transmembrane protein Aph-1 is required for stabilization of the PS1 endoproteolysis products PS1N and PS1C (Francis et al. 2002; Lee et al. 2002; Steiner et al. 2002) and that the two transmembrane protein Pen-2 is required for endoproteolysis of PS1 (Takasugi et al. 2003; Luo et al. 2003). However, recent studies have shown that Pen-2 is dispensable for endoproteolysis of PS1 (Mao et al. 2012; Holmes et al. 2014). One study also showed that NCT is not absolutely required for γ-secretase activity (Zhao et al. 2010). To further determine the role of each component of the γ-secretase complex in γ-secretase activity, we used knockout cell lines to examine the effect of deletion of each component on the processing of CTFα and CTFβ. Our data demonstrated that knockout of Pen-2, as well as NCT, almost completely blocked the processing of both CTFα and CTFβ. However, knockout of Aph-1 had no significant effect on the processing of CTFα and CTFβ, indicating Aph-1 is dispensable for APP processing. Furthermore, our results revealed that NCT is differentially required for γ-secretase-catalyzed processing of APP and Notch. In addition, our data suggest that the components essential for γ-secretase-dependent APP processing are also important for APP degradation.
Materials and methods
Cell culture
Mouse embryonic fibroblast (MEF) cells established from PS1/PS2-double knockout (PS1/2−/−) cells (Herreman et al. 2000), PS1-knockout (PS1−/−) cells (De Strooper et al. 1998), PS2-knockout (PS2−/−) cells (Herreman et al. 1999), Pen-2-Knockout (Pen2−/−) cells (Bammens et al. 2011), and wild-type mouse embryonic fibroblasts were all kindly provided by Dr. Bart De Strooper (Center for Human Genetics, Belgium). Nicastrin-knockout (NCT−/−) cells (Li et al. 2003) and Aph-1abc-triple-deficient (Aph-1−/−, deficient in all three Aph-1a, Aph-1b, and Aph-1c isoforms) cells (Chiang et al. 2012) were kindly provided by Dr. Tong Li (John Hopkins University). The wt-7 cells (N2a cells stably expressing wild-type presenilin 1 [PS1wt] along with Swedish mutant APP [APPsw]) were kindly provided by Drs. Sangram S. Sisodia and Seong- Hun Kim (University of Chicago). All cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 2 mM L-glutamine (Lonza, Walkersville, MA, USA), 100 units/mL penicillin (Lonza), and 100 µg/mL streptomycin (Lonza).
Inhibitors and reagents
Proteasome inhibitor MG132 was purchased from Peptides International (Louisville, KY, USA). Gamma-secretase inhibitors compound E and L685, 458 and proteasome inhibitor lactacystin were purchased from EMD Millipore (Billerica, MA, USA). Lysosome inhibitors chloroquine, leupeptin, and NH4Cl were purchased from Sigma (St. Louis, MO, USA). The general caspase inhibitor, benzyloxycarbonyl-Val- Ala-Asp-fluoromethylketone (Z-VAD-fmk) was purchased from Enzo Life Sciences (Farmingdale, NY, USA). Complete protease inhibitor cocktail tablets were purchased from Roche Applied Science (Indianapolis, IN, USA). Lipofectamine LTX with plus reagent was purchased from Invitrogen (Carlsbad, CA, USA).
Antibodies
Anti-PS1C, anti-NICD (#4147, which specifically recognizes the processed Notch), anti-caspase3, and anti-caspase-6 were purchased from Cell Signaling (Danvers, MA). Anti-NCT was from Sigma-Aldrich (St. Louis, MO, USA). Polyclonal antibodies anti-Aph-1aL and anti-PEN-2N were from Covance (Emeryville, CA, USA). Anti-Aph-1bc was from NOVUS (Littleton, CO, USA). Polycolonal antibody C15 was raised against the last 15 amino acids at the very C terminal of APP (Zhao et al. 2004). Anti-myc antibody, C-Myc (9E10), was purchased from Santa Cruz (Dallas, TX, USA). Anti-GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was from EMD Millipore.
Plasmids
Plasmid expressing the truncated ectodomain and myc-tagged Notch molecule (NotchΔE) containing the murine Notch-1 leader peptide (1–23 amino acids) (Kopan et al. 1996) was kindly provided by Dr. Raphael Kopan (Washington University) and Dr. Masayasu Okochi (Osaka University, Japan). The plasmid APPsw, which expresses a C-terminal myc-tagged Swedish mutant APP (APPsw) (Thinakaran et al. 1996), was kindly provided by Dr. Gopal Thinakaran (University of Chicago).
Reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR was carried out as described previously (Hao et al. 2010). Total RNA was isolated from MEF cells mentioned above using an RNeasy mini-prep kit (Qiagen, Hilden, Germany). cDNA was synthesized from 2 µg total RNA using the ThermoScipt RT-PCR kit (Invitrogen). The cDNA products were amplified using GeneAmp PCR core reagents (Applied Biosystems, Foster City, CA, USA) and a Stratagene Mx3000P thermocycler (Agilent, Santa Clara, CA, USA) with the following program: 5 min at 95°C followed by 28 cycles of denaturing at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 45 s followed by a final extension for 7 min at 72°C. The primers used were as follows: Aph1a, forward 5′-ACGGAAGATCACCCAT-3′ and reverse 5′-TGTCAGAAGGTGACTCCCA-3′; Aph1b,c, forward 5′-CCTGACGCATCTGGTGGTG-3′ and reverse 5′-GTTCCAAGATACAGGGG-3′; and NCT, forward 5′-TCTTCTCACACATGCACGCC-3′ and reverse 5′-CATGGGATCTGTGTGCATCC-3′. The PCR products were analyzed by electrophoresis on a 2% agarose gel.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed as described previously (Tan et al. 2008; Zhao et al. 2010). MEF cells were cultured for 24 h. Conditioned media (CM) were supplemented with an inhibitor cocktail (Millipore) containing AEBSF (4-[2-aminoethyl] benzenesulfonyl fluoride hydrochloride) at a final concentration of 1 mM. The CMs were analyzed with a mouse Aβ40-specific ELISA kit (Invitrogen), according to the manufacturer’s instructions.
Cell-free assay
In vitro AICD (APP intracellular domain) generation was determined by cell-free assay using the protocol reported by Tesco et al (Tesco et al. 2005a). MEF cells were grown at a density of 150,000 cells/cm2 for 24 h. Cells were scraped in 1 ml buffer A (50 mM HEPES, 150 mM NaCl, 5 mM 1,10-phenanthroline monohydrate [PNT], pH=7.4) and homogenized by passing them through 25-gauge 5/8 needles 10 times. The homogenate was centrifuged at 10,000 × g for 15 min at 4°C. The membrane fraction obtained was washed once with buffer A and centrifuged at 10,000 × g for 5 min at 4°C. Total protein was measured in the membrane fraction, and protein aliquots were incubated with 50 µl buffer B (50 mM HEPES, 150 mM NaCl, 5 mM PNT, cocktail protease inhibitor, chloroquine (10 µM), pH=7.0) for 2 h at 37°C in the presence or absence of L685, 458 to induce the production of AICD. After incubation, samples were centrifuged at 10,000 × g for 15 min at 4°C. The supernatants were collected and analyzed by Western blot using anti-APP-CTF antibody, C15.
SDS-PAGE and Western blotting
For analysis of endogenous APP processing, 10 h after splitting, cells were incubated overnight in the presence or absence of the following inhibitors compound E (5nM), L685, 458 (0.5 µM), lactacystin (10 µM), MG132 (5uM), chloroquine (10 µM), leupeptin (5 µg/ml), and NH4Cl (1mM). For analysis of the exogenous APP and Notch processing, the cells, 24 h after splitting, were transfected with plasmids expressing APPsw or NotchΔE with lipofectamine LTX. Ten hours after transfection, inhibitors were added and the cells were further incubated overnight. Cell lysis and Western blot analysis were carried out as described previously (Zhao et al. 2004). Briefly, cells were lysed with sonication for 20 s on ice in Western blot lysis buffer (50 mM Tris–HCl, pH 6.8, 8 M urea, 5% mercaptoethanol, 2% SDS, and protease inhibitor mixture). After addition of 4 × SDS sample buffer and boiling at 100°C for 7 min, samples were subjected to SDS-polyacrylamide gel electrophoresis (SDS PAGE, 16% for APP CTFs; 14% for PS1 C terminals, caspases, and GAPDH; 10% for Notch and C-Notch; 6% for APP). The membranes were probed with appropriate antibodies as described in figure legends.
Statistical analysis
Data are expressed as mean ± SEM and assessed for significance by Student's t test. When P > 0.05, differences were considered not significant∞.
All methods used are approved by University of Tennessee (Registration #309-13).
Results
Aph-1 is dispensable for γ-secretase-catalyzed processing of CTFα
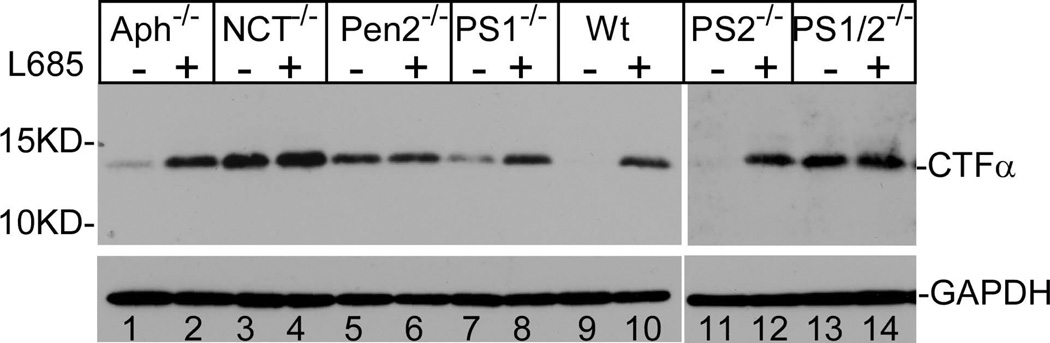
To determine the role of the components of the γ-secretase complex in APP processing activity, we examined the effects of deletion of each component of the complex on the processing of CTFα. As shown in Fig. 1a, as expected, in the absence of inhibitor, almost no CTFα was detectable in wild type (wt) cells (lane 9). However, when the cells were treated with transition state γ-secretase inhibitor L-685,458, a significant amount of unprocessed CTFα was accumulated (lane 10). As reported previously (Herreman et al. 2000), a dramatic accumulation of unprocessed CTFα was observed in the PS1 and PS2 double knockout (PS1/2−/−) cells (compare lanes 13 and 14) regardless of the presence or absence of γ-secretase inhibitor. Similarly, significant accumulation of CTFα was also observed in nicastrin-knockout (NCT−/−) cells (lanes 3 and 4) and Pen-2-knockout (Pen2−/−) cells (lanes 5 and 6) regardless of the presence or absence of γ-secretase inhibitor.
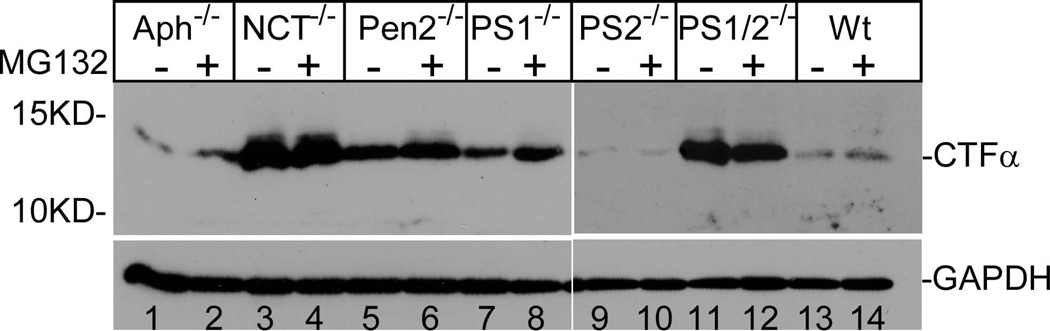
Figure 1.
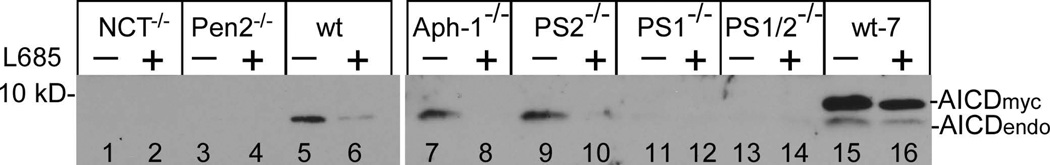
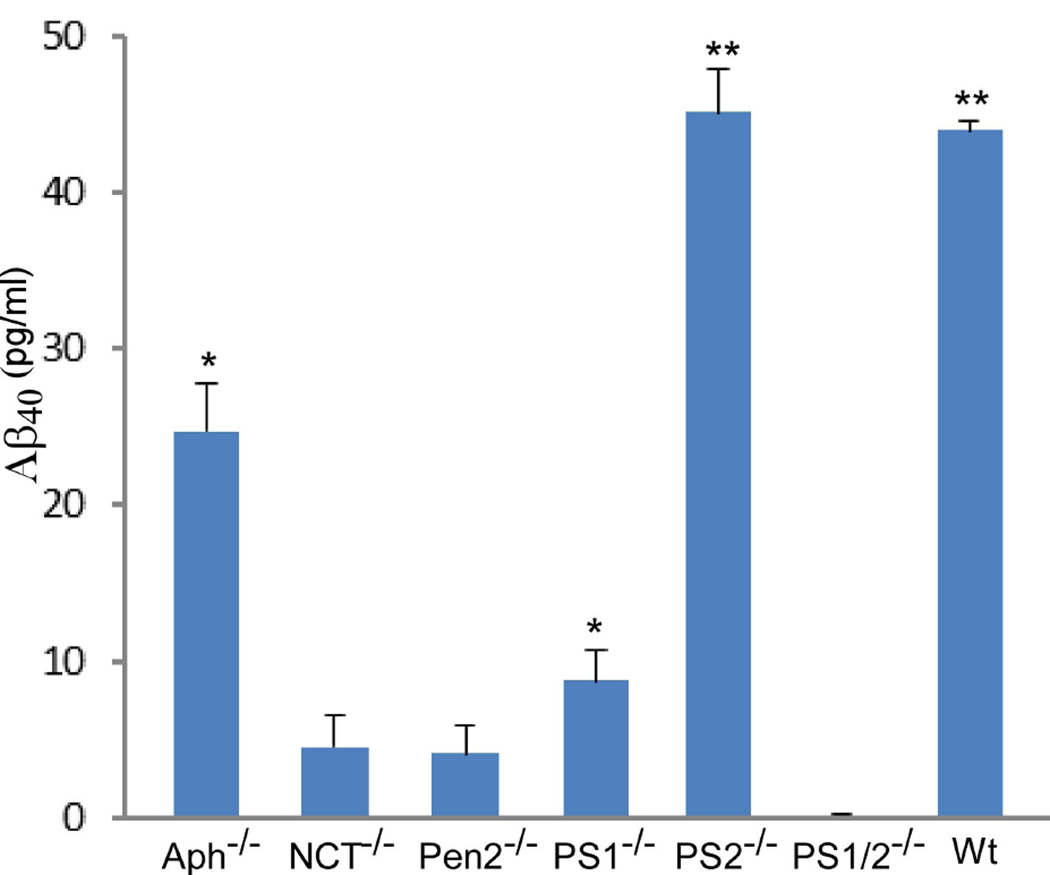
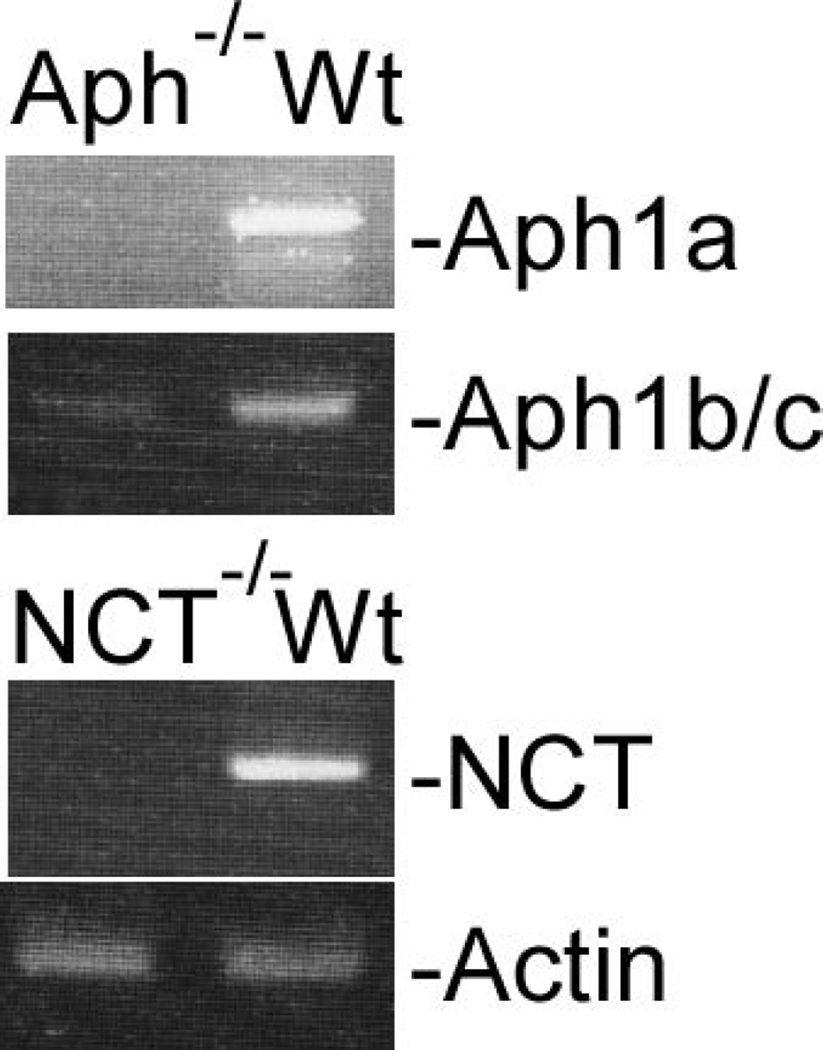
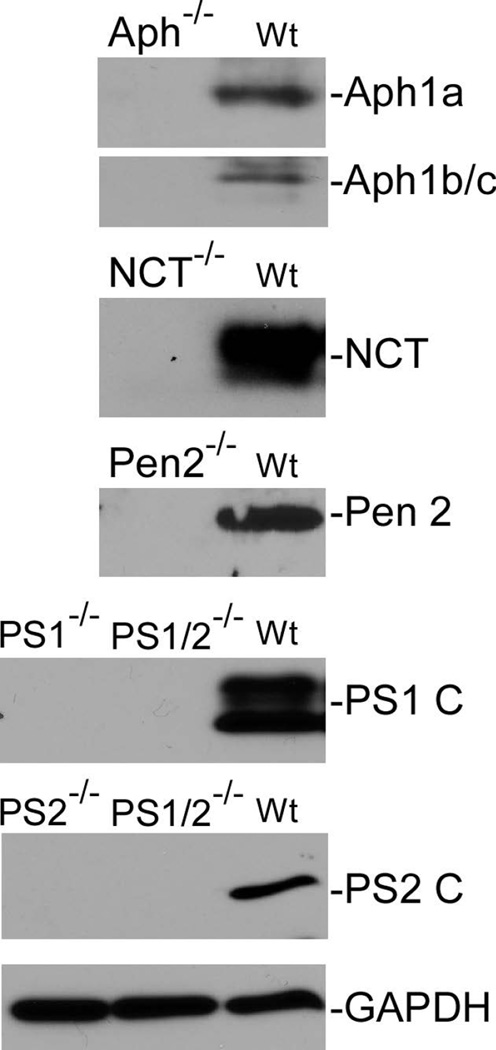
Aph-1 is dispensable for γ-secretase catalyzed APP processing. Cells were cultured in the presence and absence of γ-secretase inhibitor L-685,458 (a) or MG132 (b) overnight, lysed, and subjected to 16% SDS-PAGE and Western blot analysis using antibody C15 that was raised against the very c-terminal 15 residues of APP. The membranes were also reprobed with anti-GAPDH to indicate even loading of the samples (bottom panels). All data presented in this study are representative of at least three independent experiments. (c) Cell-free assay for in vitro generation of AICD. AICDendo: AICD produced from endogenous APP; AICDmyc: AICD produced from myc-tagged exogenous APPSw in a wt-7 stable cell line, which was used as a positive control. (d) Effect of knockout of different components of γ-secretase on Aβ formation. Aliquots of CM samples of knockout cells were subjected to ELISA to detect Aβ40. A significant amount of Aβ40 was detected in Aph-1−/− cells, as well as in wt cells. Low amount Aβ40 was also detected in PS1−/− cells, and even lower Aβ40 was detected in NCT−/− and Pen-2−/− cells. N = 3, *p < 0.01; **p < 0.001. (e) Western blot analysis of protein levels of γ-secretase components in knockout cells. (f) RT-PCR analysis of NCT and Aph-1 genes in corresponding knockout cells. Note: Since Aph-1c is the duplicate of Aph-1b in mice, the antibody against Aph-1b also detects Aph-1c, and the RT-PCR primers used are also common to both Aph-1b and Aph-1c.
However, in contrast to knockout of NCT or Pen-2, a significant decrease in the level of CTFα was detected in the Aph-1-knockout (Aph-1−/−) cells in which all three murine Aph-1 alleles–termed Aph-1a, Aph-1b, and Aph-1c–were knocked out (Fig. 1a, lane 1). More interestingly, the decrease in the level of CTFα was completely blocked by γ-secretase inhibitor (lane 2). In addition, we also observed that knockout of PS2 had almost no effect on the turnover of CTFα (lane 11) and this decrease in CTFα in PS2-knockout (PS2−/−) cells was completely inhibited by γ-secretase inhibitor (lane 12). This result indicates that knockout of PS2 did not cause significant reduction in γ-secretase activity. However, a significant amount of CTFα was detected in the PS1-knockout (PS1−/−) cells in the absence of inhibitor (lane 7), indicating a substantial reduction in γ-secretase activity.
Previous studies have reported that CTFs of APP undergo degradation by a proteasome-dependent mechanism distinct from γ-secretase (Nunan et al. 2001; Nunan et al. 2003; Skovronsky et al. 2000). To determine whether the decrease of CTFα detected in the Aph-1-knockout cells is indeed due to γ-secretase, we examined the effect of proteasome inhibitor on the turnover of CTFα. As shown in Fig. 1b, treatment of cells with proteasome inhibitor MG132 caused a slight increase in the level of CTFα in Aph-1−1− cells (compare lane 2 with lane 1). A similar result was also observed in PS1-knockout cells (compare lane 8 with lane 7) and wt cells (compare lane 14 with lane 13). However, the extent of the increase in CTFα caused by MG132 is much less than that caused by γ-secretase inhibitor (compare Fig. 1b with 1a). These results indicate that, similar to wt cells, the turnover of CTFα in the Aph-1−/− cells is mainly catalyzed by γ-secretase activity. In addition, MG132 showed no significant effect on the level of CTFα in NCT−/− cells (compare lanes 4 with lane 3), Pen-2−/− cells (compare lane 6 with lane 5), nor PS1/2−/− cells (compare lane 12 with lane 11). It was noted that no CTFβ was detected in these experiments, suggesting a possibility that the mouse endogenous APP was mostly processed via the α-secretase pathway and that the low level of CTFβ was undetectable under our experimental conditions.
If the turnover of CTFα in Aph-1−/− cells were catalyzed by γ-secretase activity rather than by random degradation, the AICD produced by γ-secretase activity would be detectable. However, AICD was not detected in the experiments shown in Fig. 1a and b, possibly due to rapid degradation of this peptide in living cells [Cupers, 2001 #7150]. Thus, we performed a cell-free assay using the procedure described previously (Tesco et al. 2005b). As shown in Fig. 1c, in the absence of γ-secretase inhibitor, a significant amount of AICD was readily detected in membrane prepared from wt (lane 5), Aph-1−/− (lane 7), and PS2−/− (lane 9) cells, and the generation of AICD in these cells was strongly inhibited by γ-secretase inhibitor L-685,458 (lanes 6, 8, and 10). Similarly, in wt-7 cells, both AICD-myc and AICDendo, produced from exogenous APP with a myc-tag and endogenous APP, respectively, were detected at very high levels (lane 15) and inhibited by L-685,458 (lane 16). However, this AICD was not detected in NCT−/−, Pen2−/−, and PS1/2−/− cells regardless of the presence or absence of γ-secretase inhibitor (lanes 1 to 4, and lanes 13 and 14). These results strongly indicate that the turnover of CTFα in Aph−/− cells is catalyzed by γ-secretase activity. AICD was hardly detected in PS1−/− cells (lanes 11 and 12), suggesting that PS1 accounts for the majority of the γ-secretase activity. To further ascertain whether APP is indeed processed by γ-secretase in Aph-1−/− cells, we performed an ELISA to determine the formation of Aβ in these cells. As shown in Fig. 1d, a large amount of Aβ40 was detected in the media of wt and PS2−/− cells. Interestingly, a significant amount of Aβ40 (> 50% of that detected in wt cells) was also detected in Aph-1−/− cells when PS1/2−/− cells were used as a negative control. This result provided further strong support to the notion that APP is indeed processed by γ-secretase activity in Aph-1−/− cells. On the other hand, only a low, but still significant, level of Aβ40 (< 20% of that detected in wt cells) was detected in PS1−/− cells, and a very low level of Aβ40 (< 8% of that detected in wt cells) was also detected in NCT−/− and Pen2−/− cells.
Aph-1c protein is undetectable in Aph-1abc-triple deficient cells under the experimental conditions
Since the Aph-1−/− cells were created by knockdown of Aph-1c in Aph-1a/b double knockout cells using shRNA technology (Chiang et al. 2012), one concern is whether the γ-secretase activity detected in Aph-1−/− cells results from incomplete knockdown of Aph-1c. To address this issue, we performed a RT-PCR assay to determine the mRNA level of Aph-1c using primers corresponding to the coding regions of Aph-1c. As controls, similar RT-PCR was also performed for Aph-1a and NCT. As shown in Fig. 1e, as expected, neither NCT mRNA nor Aph-1a mRNA was detected in NCT−/− and Aph-1−/− cells, respectively. However, as shown in the second panel of Fig. 1e, a fine PCR band was detected in Aph-1−/− cells, indicating the presence of a trace amount of residual or partially cleaved Aph-1c mRNA in Aph-1−/− cells. Thus, we further determined the protein levels of Aph-1c and other components in these knockout cells used. As shown in Fig. 1f, Western blot analysis using specific antibodies confirmed the absence of PS1, PS2, NCT, and Pen-2 as well as Aph-1 (Aph-1a, Aph-1b, and Aph-1c) proteins in the corresponding knockout cells. Specifically, the fact that antibody specific to Aph-1b/c did not detect any signal in Aph-1−/− cells suggests that the Aph-1c gene was efficiently silenced by shRNA technology.
Components of the γ-secretase complex might also play a role in regulating APP CTF degradation by proteasome and lysosome
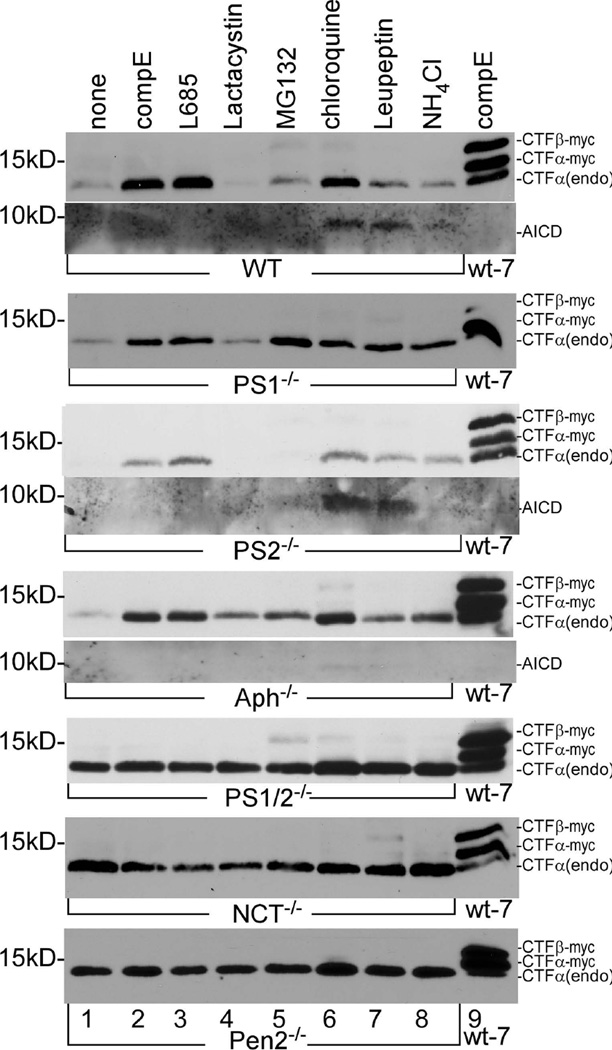
It was noted from the above experiments that treatment with proteasome inhibitor MG132 caused an increase in the level of CTFα in wt, Aph-1−/−, and PS1−/− cells. However, MG132 showed no effect on the level of CTFα in NCT−/−, Pen-2−/−, and PS1/2−/− cells. These results suggest that knockout of different components might have different effects on the proteasome-dependent turnover of CTFα. APP and its processing products have also been reported to be subjected to lysosome degradation (Eisele et al. 2007; Vingtdeux et al. 2007). Thus, next, we examined the effects of other proteasome and lysosome inhibitors on the turnover of CTFα in these knockout cells. As shown in lanes 2 and 3 of the top four panels of Fig. 2a, as expected, both of the γ-secretase inhibitors, compound E (compE) and L-685,458, caused accumulation of unprocessed CTFα in wt, PS1−/−, PS2−/−, and Aph-1−/− cells. When the cells were treated with proteasome inhibitors MG132, strong accumulation of CTFα resulted in wt, PS1−/−, and Aph-1−/− cells (lane 5), but lactacystin in comparison, caused a lesser accumulation of CTFα in PS1−/− and Aph-1−/− cells (lane 4), and CTFα was hardly detectable in wt cells (lane 4). Neither MG132 nor lactacystin had a detectable effect on the CTFα level in PS2−/− cells (Panel 3, lanes 4 and 5). When the cells were treated with the lysosome inhibitors chloroquine, leupeptin, and NH4Cl, significant accumulation of CTFα was observed in wt, PS1−/−, PS2−/−, and Aph-1−/− cells. In addition, it was noted that in the presence of lysosome inhibitors, specifically, chloroquine and leupeptin, the APP intracellular c-terminal domain (AICD) produced by γ-cleavage of CTFα become detectable in wt cells, PS2−/− cells, and to a lesser extent in Aph-1−/− cells. These results suggest that lysosome is the major site for CTFα degradation. In addition, the detection of AICD in the presence of lysosome inhibitors indicates that these lysosome inhibitors have no effect on γ-secretase catalyzed processing of CTFα. As shown in the bottom three panels of Fig. 2a, the proteasome inhibitors lactacystin and MG132 had no effect on the level of CTFα in PS1/2−/−, NCT−/−, and Pen2−/− cells. Lysosome inhibitors caused a slight increase in the level of CTFα in these cells. These results indicate that CTFα was not significantly degraded by either proteasome or lysosome activity in these cells.
Figure 2.
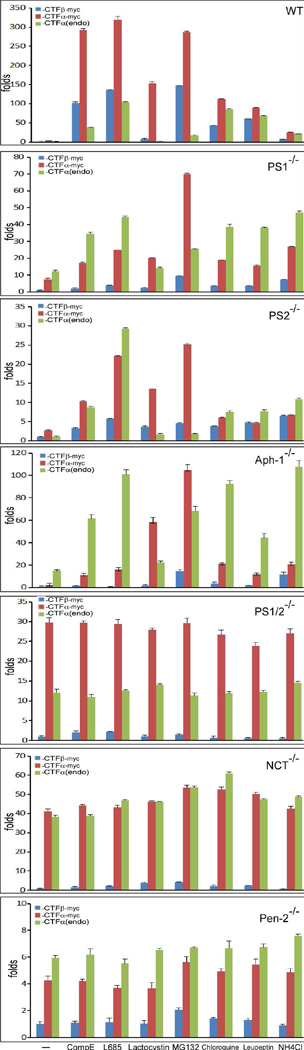
Components of the γ-secretase complex also play a role in regulating APP degradation by proteasome and lysosome. (a) Effects of γ-secretase, proteasome, and lysosome inhibitors on the accumulation of unprocessed endogenous CTFα. The first lane is the vehicle-treated control. The last lane is the sample prepared from wt-7 cells treated with γ-secretase inhibitor compound E (compE) used as standards of CTFβ-myc and CTFα-myc. (b) In lanes 3–10, cells were transfected with human APPsw expression plasmid. In lane 2, cells were transfected with unrelated protein LacZ. In lane 1, cells were mock transfected with an empty vector. Lane 11 is the sample prepared from wt-7 cells treated with compound E used as standards of CTFβ-myc and CTFα-myc. All APP CTFs were detected using C15. (c) Quantitative analysis of the formation and turnover of APP-CTFs. Results are expressed as the mean (± SD) of three independent Western blot results shown in Fig. 2B.
As mentioned above, possibly because mouse endogenous APP was mostly processed via the α-secretase pathway, the level of endogenous CTFβ was too low to be detected under our experimental conditions. To determine the effects of knockout of each γ-secretase component on the processing of CTFβ, we transiently transfected these cells with a plasmid expressing myc-tagged human Swedish mutant APP (APPsw) in the presence or absence of different inhibitors. As shown in Fig. 2b, recombinant APP was detected in all transfected cells. As shown in the top panel, in the wild type-cells, endogenous CTFα (CTFα[endo]) as well as CTFα-myc and CTFβ-myc produced from exogenous myc-tagged APPsw, were accumulated in the presence of the γ-secretase inhibitors compound E (lane 4) and L-685,458 (lane 5). Similarly, γ-secretase inhibitors caused accumulation of unprocessed CTFα-myc, and CTFα(endo) was also clearly detected in Aph-1−/−, PS2−/−, and PS1−/− cells. These results indicate that γ-secretase inhibitors had similar effects on both exogenous and endogenous APP in these cells, excepting that CTFβ-myc was hardly detected in these cells. In wt cells, the accumulation of CTFα(endo), CTFβ-myc, and CTFα-myc was also detected when cells were treated with the lysosome inhibitors chloroquine and leupeptin, and to a lesser extent with NH4Cl (lanes 8–10). However, mainly CTFα-myc and CTFβ-myc, but almost no CTFα(endo), were accumulated in the presence of proteasome inhibitors lactacystin (lane 6) and MG132 (lane 7). In PS1−/−, PS2−/−, and Aph-1−/− cells, both CTFα(endo) and CTFα-myc were detected at various levels in the presence of these proteasome and lysosome inhibitors. However, almost no CTFβ-myc was detected in these cells, with the exception of MG132-treated Aph-1−/− cells (fourth panel, lane 7). A small amount of CTFα-myc was detected in PS1−/− cells in the absence of any inhibitors (second panel, lane 3), indicating a low γ-secretase activity in these cells in comparison with that in PS2−/− cells.
It was interestingly noted that in PS1−/−, PS2−/−, and Aph-1−/− cells, treatment with proteasome inhibitors lactacystin and MG132 mainly caused accumulation of CTFα-myc (Fig. 2b, lanes 6 and 7), whereas lysosome inhibitors mostly caused accumulation of CTFα(endo) (lanes 8–10). These data revealed an interesting finding that exogenous APP was primarily degraded by proteasome, and the endogenous APP was mostly degraded by lysosome. This notion was further supported by the fact that exogenous full-length APP (both mature and immature forms) was detected at high levels in the presence of proteasome inhibitors in all cells (lanes 6 and 7). In contrast to the PS1−/−, PS2−/−, and Aph-1−/− cells, neither proteasome nor lysosome inhibitors had a significant effect on the levels of CTFα(endo) and CTFα-myc in PS1/2−/− cells (fifth panel), NCT−/− cells (sixth panel), nor Pen2−/− cells (seventh panel), indicating that APP CTFs were not significantly degraded by these organelles in these cells. A small amount of CTFβ-myc was also detected in these cells, specifically in cells treated with MG132 and lysosome inhibitors (lanes 7–10). The above results clearly indicate that the effects of proteasome and lysosome on the turnover of full-length APP and APP CTFs vary in different knockout cells.
γ-secretase-catalyzed CTFα processing in Aph-1−/− cells is independent of proteasome and lysosome activity
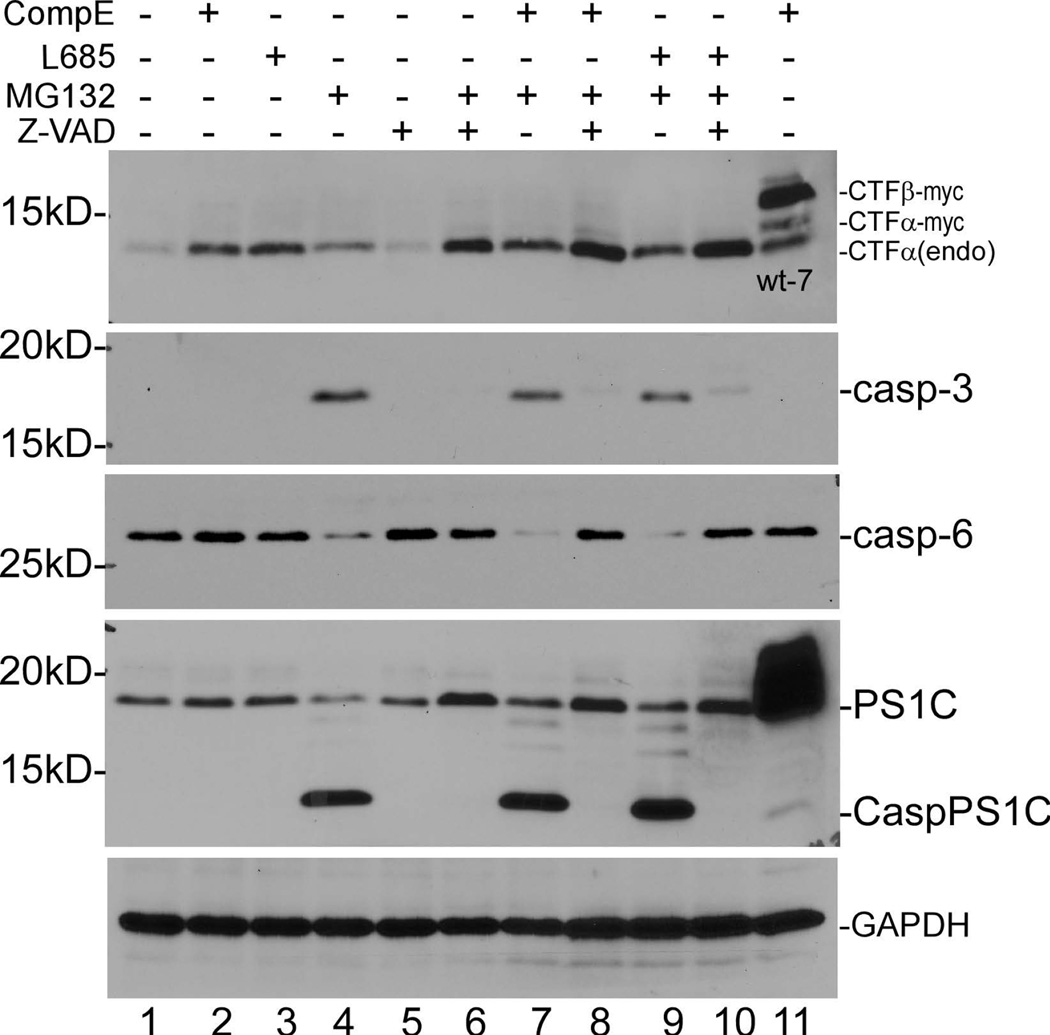
Data presented in Fig. 2a show that AICD was detected in Aph-1−/− cells as well as in wt and PS2−/− cells in the presence of lysosome inhibitors, indicating that γ-secretase activity was not affected by these lysosome inhibitors. In other words, γ-secretase-catalyzed processing of CTFα is independent of lysosome activity in these cells. To further determine whether the γ-secretase inhibitors compound E and L-685,458 caused accumulation of CTFα in Aph-1−/− cells was not due to inhibition of proteasome or lysosome activity, we performed the following experiments. As shown in Fig. 3a, the amount of CTFα accumulated in cells treated with both compound E and MG132 (lane 7) was roughly the sum of the CTFα detected in cells treated with compound E (lane 2) and MG132 (lane 4), separately. A similar result was also observed when L-685,458 was used in combination with MG132 compared with L-685,458 and MG132 alone (compare lane 9 with lanes 3 and 4). During the course of the experiments, it was noted that treatment with MG132 could induce the activation of caspase, which has been implied in the turnover of APP CTFs (Weidemann et al. 1999). This raised the question as to whether inhibition of caspase activation would lead to further accumulation of CTFα in cells treated with MG132. Indeed, a greater amount of CTFα was observed in MG132-treated cells in the presence of pan caspase inhibitor Z-VAD (compare lane 6 with lane 4). When these cells were further treated with compound E, an even greater amount of CTFα was accumulated (compare lane 8 with lane 6). A similar result was also observed when L-685,458 was added with MG132 and Z-VAD (compare lane 10 with lane 6). These results indicate that γ-secretase inhibitor and proteasome inhibitor have an additive effect on the accumulation of unprocessed CTFα through different mechanisms. Furthermore, it was noted that in addition to regular PS1C produced by normal endoproteolytic processing of PS1, a short C-terminal fragment of PS1, CaspPS1C, which was produced by caspase activity (Zeng et al. 2015), was detected in cells treated with MG132 (lanes 4, 7, and 9), and the formation of CaspPS1C was completely inhibited by the addition of pan caspase inhibitor Z-VAD (lanes 6, 8, and 10).
Figure 3.
γ-secretase, proteasome, and lysosome inhibitors have an additive effect on CTFα accumulation in Aph-1−/− cells. (a) Cells in lanes 2–10 were cultured in the presence of γ-secretase inhibitors and proteasome and caspase inhibitors either individually or in combination. Top panel, immunoblot probed with C15; second panel, immunoblot probed with anti-caspase-3 to detect the formation of the active form of caspase-3; third panel, immunoblot probed with anti-caspase-6 to determine the reduction of pro-caspase-6 due to activation; fourth panel, immunoblot probed with anti-PS1C, which reacts with both regular PS1C and the caspase-produced CaspPS1C (#5643 from Cell Signaling); the immunoblot in the fourth panel was also reprobed with anti-GAPDH to indicate relative loading of samples (bottom panel). Lane 11 is the sample from wt-7 cells cultured in the presence of compound E. (b) Cells in lanes 2–10 were cultured in the presence of γ-secretase inhibitors and lysosome inhibitors either individually or in combination. Top panel, immunoblot probed with C15; bottom panel, this immunoblot was reprobed with anti-GAPDH. Lane 11 is the sample from wt-7 cells cultured in the presence of compound E.
Next, we examined the additive effect of γ-secretase inhibitors and lysosome inhibitors on the accumulation of unprocessed CTFα. As shown in Fig. 3b, the amount of CTFα accumulated in the cells treated with both compound E and chloroquine (lane 6) was roughly the sum of the CTFα detected in cells treated with compound E (lane 2) and chloroquine (lane 4), separately. A similar result was also observed when cells were treated with L-685,458 and chloroquine (compare lane 7 with lanes 3 and 4). Likewise, leupeptin exhibited a similar additive effect on CTFα accumulation when used in combination with compound E (compare lane 8 with lanes 2 and 5) and L685, 458 (compare lane 9 with lanes 3 and 5). These data indicate that γ-secretase inhibitor-caused accumulation of CTFα in Aph-1−/− cells is not due to inhibition of proteasome or lysosome, i. e., γ-secretase-catalyzed CTFα processing in Aph-1−/− cells is independent of proteasome and lysosome activity.
Aph-1, as well as nicastrin is, dispensable for γ-secretase-catalyzed processing of Notch
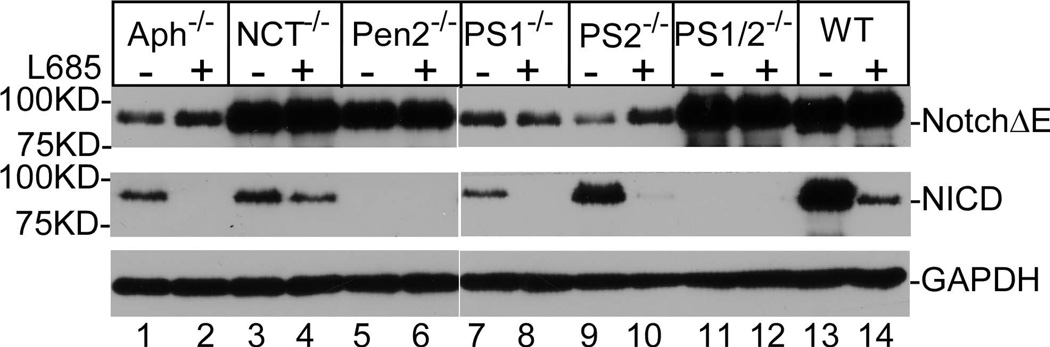
Data presented above demonstrate that Aph-1 is not absolutely required for γ-secretase-catalyzed APP CTF processing, while NCT and Pen-2 are crucially essential for this process. In addition to APP, Notch is another well-characterized substrate of γ-secretase. We next examined the effect of knockout of different components of the γ-secretase complex on the processing of Notch. To do so, cells were transfected with a plasmid expressing NotchΔE, the ectodomain-truncated and myc-tagged Notch containing the murine Notch-1 leader peptide (1–23 amino acids) (Kopan et al. 1996) in the presence or absence of γ-secretase inhibitor L-685,458. As shown in Fig. 4a, recombinant NotchΔE was detected with anti-myc antibody at various levels in wild-type and knockout cells, possibly due to different transfection efficiency. As shown in the middle panel, NICD, which is produced by γ-secretase from NotchΔE, was detected in wild-type cells (lane 13), PS2−/− cells (lane 9), and PS1−/− cells (lane 7), Aph-1−/− cells (lane 1), and NCT−/− cells (lane 3), and the formation of this NICD was strongly inhibited by the addition of L-685,458 (lanes 2, 4, 8, 10, and 14). However, this NICD was not detected in PS1/2−/− cells (lane 11) nor Pen2−/− cells (lane 5). These results revealed an interesting finding that, under our experimental conditions, NCT is crucially essential for γ-secretase-catalyzed APP CTFs processing, but is not absolutely required for γ-secretase-catalyzed Notch processing.
Figure 4.
Aph-1 and nicastrin are not essential for γ-secretase catalyzed processing of Notch. (a) Aph-1−/− cells were transfected with a plasmid expressing N-terminal truncated Notch with a C-terminal myc tag. Top panel, immunoblot probed with anti-myc to detect the unprocessed recombinant Notch. Middle panel, immunoblot probed with antibody, which specifically recognizes the N-terminus of NICD generated by γ-secretase processing. Bottom panel, immunoblot in the middle panel reprobed with anti-GAPDH. (b) Proteasome and lysosome have no significant effect on Notch metabolism. Top panel, immunoblot probed with anti-myc to determine the levels of NotchΔE in the presence of different inhibitors; middle panel, immunoblot probed with anti-NICD; bottom panel, immunoblot in middle panel was reprobed with anti-GAPDH.
Generation of NICD from NotchΔE is not affected by proteasome and lysosome inhibitors
Data presented in Fig. 2 demonstrate that CTFα was also degraded by proteasome and lysosome in a γ-secretase-independent mechanism. Next, we determined whether Notch is also subjected to proteasome and/or lysosome degradation and whether proteasome and lysosome inhibitors have any effect on NICD formation. As shown in the middle panel of Fig. 4b, γ-secretase inhibitors compound E (lane 4) and, specifically, L-685,458 (lane 5) strongly inhibited the formation of NICD from NotchΔE. However, the level of NICD in proteasome inhibitors-treated cells was slightly increased (lanes 6 and 7), likely due to the protection of NICD from degradation, while the lysosome inhibitors showed no effect on the generation of NICD (lanes 8–10). In addition, the level of unprocessed NotchΔE was also slightly increased in proteasome inhibitor-treated cells (top panel, lane 6 and 7), suggesting that, though to lesser extent, NotchΔE also underwent proteasome degradation. Taking together, these data suggest that both NotchΔE and NICD undergo proteasome degradation, but the proteasome and lysosome inhibitors have no effect on γ-secretase-catalyzed processing of Notch.
Recovery of PS1C does not necessarily restore the γ-secretase activity toward APP in NCT−/− cells
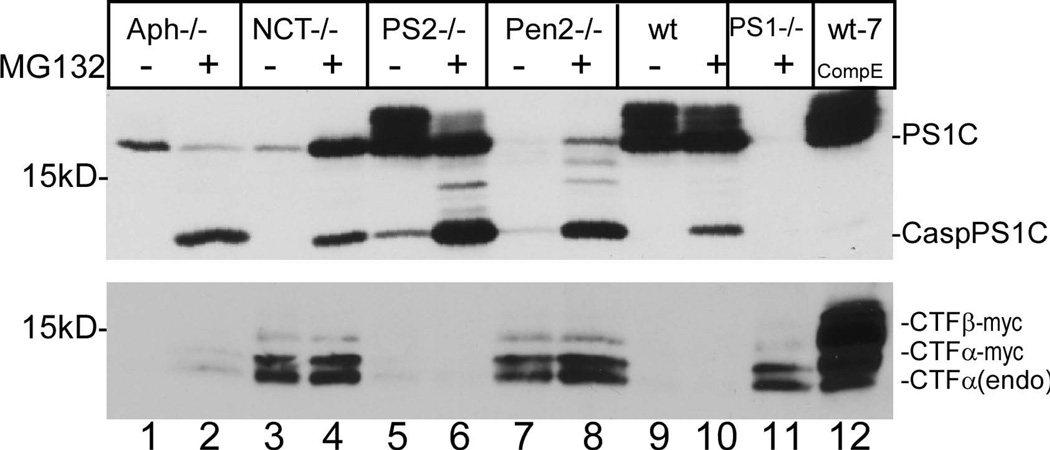
Previous study revealed that Pen-2, Aph-1, and NCT are not necessary for endoproteolytic processing of PS1, but are required for stabilization of the PS1 endoproteolytic processing products PS1N and PS1C (Mao et al. 2012). Thus, it is speculated that the loss of γ-secretase activity toward CTFα and CTFβ might have resulted from the instability of endoproteolytic products of PS1 in NCT−/− and Pen2−/− cells. As shown in the top panel of Fig. 5, in the absence of MG132, PS1C was detected in wt cells (lane 9), in PS2−/− cells (lane 5), and, to a lesser but significant extent, in Aph-1−/− cells (lane 1). A very low level of PS1C was detected in NCT−/− cells at (lane 3), and only a trace amount of PS1C was detected in Pen-2 cells (lane 7). As expected, no PS1C was detected in PS1−/− cells (lane 11). This result confirmed again that Pen-2 is crucial for stabilizing PS1C. This result also revealed that Aph-1 is less important for stabilizing the endoproteolytic products of PS1.
Figure 5.
Recovery of PS1C does not necessarily restore the γ-secretase activity toward APP in NCT−/− cells. Knockout cells were cultured in the presence or absence of MG132. Up panel, immunoblot probed with anti-PS1C that recognizes both regular and caspase produced PS1C; bottom panel, immunoblot probed with C15. Lane 12 is the sample prepared from wt-7 cells treated with compound E used as standards of CTFβ-myc and CTFα-myc.
When the cells were treated with MG132, a significant decrease in the level of PS1C and a concomitant significant increase in the level of CaspPS1C produced by caspase activity were detected in wt cells (lane 10), PS2−/− cells (lane 6), and Aph-1−/− cells (lane 2). In the presence of MG132, CaspPS1C was also detected in NCT−/− and Pen2−/− cells (lanes 4 and 8). However, in contrast to wt, PS2−/−, and Aph-1−/− cells, the increase in CaspPS1C was not associated with a decrease, but rather an increase in the regular PS1C in NCT−/− cells (compare lane 4 with lane 3) and Pen-2−/− cells (compare lane 8 with lane 7). Interestingly, as shown in the bottom panel, the high levels of unprocessed CTFα(endo), CTFα-myc, and CTFβ in NCT−/− and Pen2−/− cells were not affected by the addition of MG132. These results indicate that recovery of PS1C does not necessarily restore γ-secretase activity toward CTFα and CTFβ. In other words, Pen-2 and, specifically, NCT, as essential components of γ-secretase, must play a direct role in γ-secretase activity in addition to their roles in stabilizing PS1 proteolytic products. In this regard, NCT has been proposed to function as a substrate receptor (Shah et al. 2005).
Discussion
Previous studies using reconstitution and knockdown approaches have suggested that the four proteins, presenilin (PS1 or PS2), NCT, Aph-1, and Pen-2, are necessary and sufficient for γ-secretase activity (Edbauer et al. 2003; Kimberly et al. 2003; Takasugi et al. 2003). However, this view was challenged by a recent study showing that Notch was processed in a γ-secretase-dependent manner in NCT-deficient cells, suggesting that NCT is not absolutely required for γ-secretase activity (Zhao et al. 2010). In the current study, by taking advantage of the availability of all cell lines deficient in one of the four components of the γ-secretase complex, we performed a series of experiments to attempt to address this controversial issue. Using these cells, our results demonstrated that knockout of PS2 had almost no effect on APP CTFs processing and that, in contrast, knockout of PS1 strongly inhibited APP CTF processing as determined by the turnover of CTFα, as well as the formation of AICD and Aβ40. These observations confirmed that PS1 accounts for the majority of γ-secretase activity that catalyzes the processing of APP CTFs. In addition, our results revealed several interesting findings. First, our data demonstrate that, in contrast to NCT−/− and Pen-2−/− cells, in which no significant CTFα turnover and only a small amount of Aβ40 was detected, similar to wt cells, a low level of CTFα and significant amount of AICD were detected in Aph-1−/− cells. Also, the turnover of CTFα and the formation of AICD were strongly inhibited by γ-secretase inhibitor, suggesting that the turnover of CTFα and the formation of AICD in Aph-1−/− cells, as well as in wt cells, were catalyzed by γ-secretase. In addition, another γ-secretase substrate, Notch, was also processed in a γ-secretase-dependent manner in Aph-1−/− cells. Furthermore, based on the levels of Aβ40 determined by ELISA, it is assumed that over 50% of γ-secretase activity was retained in Aph-1−/− cells. Although it cannot be ruled out that the trace amount of the residual Aph-1c, which was not detectable at the protein level under our experimental conditions, may contribute to a small portion of the γ-secretase activity in Aph-1−/− cells, all these observations strongly suggest that Aph-1 is not absolutely required for γ-secretase activity. In addition, albeit at a very low level, the detection of Aβ40 by ELISA in NCT−/− and Pen-2−/− cells suggests that deletion of one of these two components does not completely abolish γ-secretase activity. Thus, it is very likely that Aph-1, NCT, and Pen-2 are all required for achieving maximal γ-secretase activity; however, Aph-1 is less crucial than NCT and Pen-2 for the enzymatic activity in this γ-secretase complex.
It is proposed that γ-secretase harbors both endopeptidase-like and carboxypeptidase-like activities, catalyzing a series of sequential cleavages of APP and leading to the generation of Aβ peptide. In this model, APP is first cleaved at the ε-cleavage site by endopeptidase-like activity to release the APP intracellular c-terminal domain, AICD, and generate the membrane-bound, long Aβ49 peptide, which is further sequentially chopped down roughly every three residues by carboxypeptidase-like activity to produce the secreted Aβ40 and Aβ42 and other minor, shorter Aβ species (Xu 2009). Previous studies suggest that Aph-1 might function as a scaffold involved in γ-secretase complex assembly and maturation (LaVoie et al. 2003; Luo et al. 2003) and in the binding of substrate (Chen et al. 2010; Mao et al. 2012). In determining the specific roles of different isoforms of Aph-1 in γ-secretase-catalyzed APP processing, recent studies further suggest that Aph-1 mainly affects the carboxypeptidase-like activity that catalyzes the sequential cleavages following the initial cleavage at the ε-site and determines the C-termini of Aβ species; specifically, γ-secretase complexes containing the Aph-1b isoform favor the generation of longer Aβ peptides (Serneels et al. 2005; Serneels et al. 2009; Acx et al. 2014). This notion might provide justification for our finding that Aph-1 is dispensable for the endopeptidase-like activity of γ-secretase that catalyzes the initial cleavage of CTFs at the ε-site, which is a decisive step in γ-secretase-catalyzed APP processing (Xu 2009).
The second important finding of the current study is the differential requirement for NCT in γ-secretase-catalyzed processing of APP and Notch. To elucidate the specific function of NCT, a well-designed study revealed that the extracellular domain of NCT is essential for recognition of the substrate of γ-secretase, suggesting that NCT functions as a receptor of substrate (Shah et al. 2005). However, a recent study showing that cells deficient in NCT were capable of processing Notch and, to a lesser extent, APP in a γ-secretase-dependent manner raised a question as to whether NCT is absolutely required for γ-secretase activity (Zhao et al. 2010). Using the same NCT−/− cells and the same truncated Notch-expressing plasmid as used in Zhao et al’s study, our results revealed a similar finding that Notch was processed by γ-secretase activity in the absence of NCT. In addition, our results revealed that Aph-1 was also not absolutely required for Notch processing. However, in contrast to the previous study, our data demonstrate that knockout of NCT completely abolished γ-secretase-catalyzed processing of CTFα and CTFβ produced from both endogenous and recombinant APP.
These controversial observations might have resulted from the use of different experimental systems. Specifically, in the previous study, a transiently-expressed truncated APP (C99), an artificial CTFβ, was used as a γ-secretase substrate to determine the effect of knockout of NCT on the formation of AICD from C99. In contrast, in the current study, we examined the processing of CTFα and CTFβ produced either from endogenous APP or recombinant full-length APP. After synthesis, full-length APP undergoes multiple post-translational modifications including N- and O-glycosylation, phosphorylation, and tyrosine sulphation, and these modifications not only affect the trafficking but also the processing of APP along the secretory pathway as well as the endocytotic pathway (Jiang et al. 2014). It is not known whether the overexpressed C99 also undergoes similar post-translational modification and is processed at the same subcellular locations as full-length APP. Whether possible differences in post-translational modification and trafficking may account for the discrepancy between results of the current study and that reported by Zhao et al awaits further investigation. Nevertheless, the data presented in this study strongly suggest that NCT is crucially essential for γ-secretase-catalyzed processing of CTFα and CTFβ produced from full-length APP, but that NCT is not absolutely required for Notch processing. Supporting our finding, a recent study reported that mutations in NCT differentially affect Aβ production and Notch processing (Pamrén et al. 2011). Thus, this differential requirement for NCT in γ-secretase-catalyzed processing of APP and Notch suggests NCT as a therapeutic target for developing a strategy to restrict Aβ formation in AD without impairing Notch signaling.
The third notable finding of the current study is that components of the γ-secretase complex essential for γ-secretase-catalyzed APP processing are also important for proteasome- and lysosome-dependent degradation of APP derivatives. Previous studies have reported that, in addition to γ-secretase-catalyzed processing, APP and CTFs of APP are also subjected to proteasome and lysosome degradation (Nunan et al. 2001; Skovronsky et al. 2000; Vingtdeux et al. 2007; Watanabe et al. 2012; Wang et al. 2015). In the current study, as shown in Fig. 1 and 2, our data demonstrate that proteasome inhibitor MG132 and, specifically, lysosome inhibitors chloroquine, leupeptin, and NH4Cl caused marked accumulation of unprocessed APP CTFs in wild-type cells. A similar effect of these inhibitors on the accumulation of APP CTFs was also observed in PS1−/−, PS2−/−, and Aph-1−/− cells, which all expressed the γ-secretase activity that catalyzes the processing of APP CTFs. However, the effects of these inhibitors on the accumulation of the APP CTFs was less significant in PS1/2−/−, NCT−/−, and Pen2−/− cells, in which no γ-secretase-catalyzed APP processing was observed. These findings strongly indicate that presenilin (PS1 or PS2), NCT, and Pen2, which are essential for γ-secretase-catalyzed APP processing, are also important for proteasome- and lysosome-dependent degradation of APP CTFs. One possibility is that γ-secretase activity is involved in the proteasome- and lysosome-dependent degradation of APP CTFs. However, this is very unlikely in light of the fact that γ-secretase inhibitors and the proteasome and lysosome inhibitors exhibited additive effects on the accumulation of APP CTFs. Recent studies reported that presenilin is necessary for efficient protein degradation by lysosome in a γ-secretase-independent manner (Lee et al. 2010; Coen et al. 2012; Neely et al. 2011; Zhang et al. 2012). In this regard, it is noteworthy that our results suggest that lysosome plays a major role in degradation of APP CTFs. Therefore, the inefficient degradation of APP CTFs in PS1/2−/− cells is likely due to impaired lysosome function caused by deficiency of presenilin. Since NCT and Pen-2 are essential for stabilizing presenilin (Mao et al. 2012), the ineffective lysosomal degradation of APP CTFs in NCT−/− and Pen2−/− cells might have resulted from the instability of presenilin in these cells. It is also noted that the level of PS1C in Aph-1−/− cells is much higher than that in NCT−/− and Pen2−/− cells, and this might account for the fact that lysosomal degradation of APP CTFs was observed in Aph-1−/− cells. However, it cannot be ruled out that NCT and Pen-2 may be directly involved in PS1-regulated lysosome function rather than simply stabilizing PS1C. In addition, our results strongly suggest that endogenous and exogenous APPs undergo degradation by different mechanisms, i.e., endogenous APP mainly undergoes lysosome-dependent degradation, whereas, exogenously expressed APP is primarily degraded by proteasome.
Acknowledgements
We thank Dr. Bart De Strooper (Center for Human Genetics, K.U. Leuven, Herestraat 49, B 3000 Leuven, Belgium) for providing the presenilin-knockout and Pen-2-knockout cell lines. We thank Dr. Tong Li (John Hopkins University, Baltimore, MD, USA) for providing the nicastrin-knockout and Aph-1-knockout cell lines. We also thank Ms. Misty R. Bailey for her critical reading of the manuscript. This work was supported, in whole or in part, by National Institutes of Health Grants R181741110 and R21AG039596 (to X. X.) and 0355339B (to M.-Z. C.). This work was also supported by an Alzheimer’s Association grant, a grant from the American Health Assistance Foundation (to X. X.) and by the University of Tennessee Center of Excellence in Livestock Diseases and Human Health (to X. X.).
Abbreviations used
- AD
Alzheimer’s disease
- Aβ
amyloid beta
- AICD
amyloid precursor protein intracellular domain
- Aph-1
anterior pharynx-defective 1
- Aph-1−/−
Aph-1-knockout
- APP
amyloid precursor protein
- APPsw
Swedish mutant amyloid precursor protein
- CTF
c-terminal fragment
- CTFα(endo)
endogenous CTFα
- NCT
nicastrin
- NCT−/−
nicastrin knockout
- NotchΔE
n-terminal truncated Notch
- Pen-2
presenilin enhancer 2
- Pen2−/−
Pen-2-knockout
- PS1
presenilin-1
- PS1−/−
presenilin-1 knockout
- PS1/2−/−
presenilin1/2 double knockout
- wt
wild-type
- PS2
presenilin-2
- PS2−/−
presenilin-2 knockout
Footnotes
conflict of interest disclosure
The authors declare no conflicts of interest.
References
- Acx H, Chávez-Gutiérrez L, Serneels L, Lismont S, Benurwar M, Elad N, De Strooper B. Signature Amyloid β Profiles Are Produced by Different γ-Secretase Complexes. Journal of Biological Chemistry. 2014;289:4346–4355. doi: 10.1074/jbc.M113.530907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bammens L, Chavez-Gutierrez L, Tolia A, Zwijsen A, De Strooper B. Functional and topological analysis of Pen-2, the fourth subunit of the gamma-secretase complex. The Journal of biological chemistry. 2011;286:12271–12282. doi: 10.1074/jbc.M110.216978. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen AC, Guo LY, Ostaszewski BL, Selkoe DJ, LaVoie MJ. Aph-1 Associates Directly with Full-length and C-terminal Fragments of γ-Secretase Substrates. Journal of Biological Chemistry. 2010;285:11378–11391. doi: 10.1074/jbc.M109.088815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang P-M, Fortna RR, Price DL, Li T, Wong PC. Specific domains in anterior pharynx-defective 1 determine its intramembrane interactions with nicastrin and presenilin. Neurobiology of Aging. 2012;33:277–285. doi: 10.1016/j.neurobiolaging.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen K, Flannagan RS, Baron S, et al. Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. The Journal of Cell Biology. 2012;198:23–35. doi: 10.1083/jcb.201201076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- Dries DR, Yu G. Assembly, maturation, and trafficking of the gamma-secretase complex in Alzheimer's disease. Current Alzheimer research. 2008;5:132–146. doi: 10.2174/156720508783954695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of [gamma]-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- Eisele YS, Baumann M, Klebl B, Nordhammer C, Jucker M, Kilger E. Gleevec Increases Levels of the Amyloid Precursor Protein Intracellular Domain and of the Amyloid-β–degrading Enzyme Neprilysin. Molecular Biology of the Cell. 2007;18:3591–3600. doi: 10.1091/mbc.E07-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler WP, Kimberly WT, Ostaszewski BL, et al. Transition-state analogue inhibitors of gamma-secretase bind directly to presenilin-1. Nat Cell Biol. 2000;2:428–434. doi: 10.1038/35017062. [DOI] [PubMed] [Google Scholar]
- Francis R, McGrath G, Zhang J, et al. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell. 2002;3:85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- Hao F, Tan M, Wu DD, Xu X, Cui MZ. LPA induces IL-6 secretion from aortic smooth muscle cells via an LPA1-regulated, PKC-dependent, and p38alpha-mediated pathway. American journal of physiology. Heart and circulatory physiology. 2010;298:H974–H983. doi: 10.1152/ajpheart.00895.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Herreman A, Hartmann D, Annaert W, et al. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc Natl Acad Sci U S A. 1999;96:11872–11877. doi: 10.1073/pnas.96.21.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreman A, Serneels L, Annaert W, Collen D, Schoonjans L, De Strooper B. Total inactivation of gamma-secretase activity in presenilin-deficient embryonic stem cells. Nat Cell Biol. 2000;2:461–462. doi: 10.1038/35017105. [DOI] [PubMed] [Google Scholar]
- Holmes O, Paturi S, Selkoe DJ, Wolfe MS. Pen-2 Is Essential for γ-Secretase Complex Stability and Trafficking but Partially Dispensable for Endoproteolysis. Biochemistry. 2014;53:4393–4406. doi: 10.1021/bi500489j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Li Y, Zhang X, Bu G, Xu H, Zhang YW. Trafficking regulation of proteins in Alzheimer's disease. Mol Neurodegener. 2014;9:6. doi: 10.1186/1750-1326-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci U S A. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Schroeter EH, Weintraub H, Nye JS. Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proceedings of the National Academy of Sciences. 1996;93:1683–1688. doi: 10.1073/pnas.93.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh S, Theuns J, Van Broeck B, et al. Mean age-of-onset of familial alzheimer disease caused by presenilin mutations correlates with both increased Aβ42 and decreased Aβ40. Human Mutation. 2006;27:686–695. doi: 10.1002/humu.20336. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Fraering PC, Ostaszewski BL, Ye W, Kimberly WT, Wolfe MS, Selkoe DJ. Assembly of the gamma-secretase complex involves early formation of an intermediate subcomplex of Aph-1 and nicastrin. J Biol Chem. 2003;278:37213–37222. doi: 10.1074/jbc.M303941200. [DOI] [PubMed] [Google Scholar]
- Lee J-H, Yu WH, Kumar A, et al. Lysosomal Proteolysis and Autophagy Require Presenilin 1 and Are Disrupted by Alzheimer-Related PS1 Mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SF, Shah S, Li H, Yu C, Han W, Yu G. Mammalian APH-1 interacts with presenilin and nicastrin and is required for intramembrane proteolysis of amyloid-beta precursor protein and Notch. J Biol Chem. 2002;277:45013–45019. doi: 10.1074/jbc.M208164200. [DOI] [PubMed] [Google Scholar]
- Li T, Ma G, Cai H, Price DL, Wong PC. Nicastrin is required for assembly of presenilin/gamma-secretase complexes to mediate Notch signaling and for processing and trafficking of beta-amyloid precursor protein in mammals. J Neurosci. 2003;23:3272–3277. doi: 10.1523/JNEUROSCI.23-08-03272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Xu M, Lai MT, et al. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- Luo WJ, Wang H, Li H, et al. PEN-2 and APH-1 coordinately regulate proteolytic processing of presenilin 1. J Biol Chem. 2003;278:7850–7854. doi: 10.1074/jbc.C200648200. [DOI] [PubMed] [Google Scholar]
- Mao G, Cui MZ, Li T, Jin Y, Xu X. Pen-2 is dispensable for endoproteolysis of presenilin 1, and nicastrin-Aph subcomplex is important for both gamma-secretase assembly and substrate recruitment. Journal of Neurochemistry. 2012;123:837–844. doi: 10.1111/jnc.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely KM, Green KN, LaFerla FM. Presenilin Is Necessary for Efficient Proteolysis through the Autophagy – Lysosome System in a γ-Secretase-Independent Manner. The Journal of Neuroscience. 2011;31:2781–2791. doi: 10.1523/JNEUROSCI.5156-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunan J, Shearman MS, Checler F, Cappai R, Evin G, Beyreuther K, Masters CL, Small DH. The C-terminal fragment of the Alzheimer's disease amyloid protein precursor is degraded by a proteasome-dependent mechanism distinct from γ-secretase. European Journal of Biochemistry. 2001;268:5329–5336. doi: 10.1046/j.0014-2956.2001.02465.x. [DOI] [PubMed] [Google Scholar]
- Nunan J, Williamson NA, Hill AF, Sernee MF, Masters CL, Small DH. Proteasome-mediated degradation of the C-terminus of the Alzheimer's disease β-amyloid protein precursor: Effect of C-terminal truncation on production of β-amyloid protein. Journal of Neuroscience Research. 2003;74:378–385. doi: 10.1002/jnr.10646. [DOI] [PubMed] [Google Scholar]
- Pamrén A, Wanngren J, Tjernberg LO, Winblad B, Bhat R, Näslund J, Karlström H. Mutations in Nicastrin Protein Differentially Affect Amyloid β-Peptide Production and Notch Protein Processing. Journal of Biological Chemistry. 2011;286:31153–31158. doi: 10.1074/jbc.C111.235267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serneels L, Dejaegere T, Craessaerts K, et al. Differential contribution of the three Aph1 genes to γ-secretase activity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1719–1724. doi: 10.1073/pnas.0408901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serneels L, Van Biervliet J, Craessaerts K, et al. γ-Secretase Heterogeneity in the Aph1 Subunit: Relevance for Alzheimer’s Disease. Science. 2009;324:639–642. doi: 10.1126/science.1171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Lee SF, Tabuchi K, et al. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Skovronsky DM, Pijak DS, Doms RW, Lee VMY. A Distinct ER/IC γ-Secretase Competes with the Proteasome for Cleavage of APP†. Biochemistry. 2000;39:810–817. doi: 10.1021/bi991728z. [DOI] [PubMed] [Google Scholar]
- Steiner H, Winkler E, Edbauer D, Prokop S, Basset G, Yamasaki A, Kostka M, Haass C. PEN-2 is an integral component of the gamma-secretase complex required for coordinated expression of presenilin and nicastrin. J Biol Chem. 2002;277:39062–39065. doi: 10.1074/jbc.C200469200. [DOI] [PubMed] [Google Scholar]
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- Tan J, Mao G, Cui MZ, Kang SC, Lamb B, Wong BS, Sy MS, Xu X. Effects of gamma-secretase cleavage-region mutations on APP processing and Abeta formation: interpretation with sequential cleavage and alpha-helical model. Journal of Neurochemistry. 2008;107:722–733. doi: 10.1111/j.1471-4159.2008.05643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesco G, Ginestroni A, Hiltunen M, Kim M, Dolios G, Hyman BT, Wang R, Berezovska O, Tanzi RE. APP substitutions V715F and L720P alter PS1 conformation and differentially affect Abeta and AICD generation. Journal of neurochemistry. 2005a;95:446–456. doi: 10.1111/j.1471-4159.2005.03381.x. [DOI] [PubMed] [Google Scholar]
- Tesco G, Ginestroni A, Hiltunen M, Kim M, Dolios G, Hyman BT, Wang R, Berezovska O, Tanzi RE. APP substitutions V715F and L720P alter PS1 conformation and differentially affect Aβ and AICD generation. Journal of Neurochemistry. 2005b;95:446–456. doi: 10.1111/j.1471-4159.2005.03381.x. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Teplow DB, Siman R, Greenberg B, Sisodia SS. Metabolism of the "Swedish" amyloid precursor protein variant in neuro2a (N2a) cells. Evidence that cleavage at the "beta-secretase" site occurs in the golgi apparatus. J Biol Chem. 1996;271:9390–9397. doi: 10.1074/jbc.271.16.9390. [DOI] [PubMed] [Google Scholar]
- Vingtdeux V, Hamdane M, Bégard S, Loyens A, Delacourte A, Beauvillain J-C, Buée L, Marambaud P, Sergeant N. Intracellular pH regulates amyloid precursor protein intracellular domain accumulation. Neurobiology of Disease. 2007;25:686–696. doi: 10.1016/j.nbd.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Wang H, Sang N, Zhang C, Raghupathi R, Tanzi RE, Saunders A. Cathepsin L Mediates the Degradation of Novel APP C-Terminal Fragments. Biochemistry. 2015;54:2806–2816. doi: 10.1021/acs.biochem.5b00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Hikichi Y, Willuweit A, Shintani Y, Horiguchi T. FBL2 Regulates Amyloid Precursor Protein (APP) Metabolism by Promoting Ubiquitination-Dependent APP Degradation and Inhibition of APP Endocytosis. The Journal of Neuroscience. 2012;32:3352–3365. doi: 10.1523/JNEUROSCI.5659-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Paliga K, Dürrwang U, Reinhard FBM, Schuckert O, Evin G, Masters CL. Proteolytic Processing of the Alzheimer’s Disease Amyloid Precursor Protein within Its Cytoplasmic Domain by Caspase-like Proteases. Journal of Biological Chemistry. 1999;274:5823–5829. doi: 10.1074/jbc.274.9.5823. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and [gamma]-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. Therapeutic strategies for Alzheimer's disease. Nat Rev Drug Discov. 2002;1:859–866. doi: 10.1038/nrd938. [DOI] [PubMed] [Google Scholar]
- Xu X. γ-Secretase Catalyzes Sequential Cleavages of the AβPP Transmembrane Domain. Journal of Alzheimer's Disease. 2009;16:211–224. doi: 10.3233/JAD-2009-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Hu C, Zhang F, Xu DC, Cui M-Z, Xu X. Cellular FLICE-like Inhibitory Protein (c-FLIP) and PS1-associated Protein (PSAP) Mediate Presenilin 1-induced γ-Secretase-dependent and -independent Apoptosis, Respectively. Journal of Biological Chemistry. 2015;290:18269–18280. doi: 10.1074/jbc.M115.640177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Garbett K, Veeraraghavalu K, Wilburn B, Gilmore R, Mirnics K, Sisodia SS. A role for presenilins in autophagy revisited: normal acidification of lysosomes in cells lacking PSEN1 and PSEN2. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:8633–8648. doi: 10.1523/JNEUROSCI.0556-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li Y, Xu H, Zhang YW. The gamma-secretase complex: from structure to function. Front Cell Neurosci. 2014;8:427. doi: 10.3389/fncel.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Liu Z, Ilagan MX, Kopan R. Gamma-secretase composed of PS1/Pen2/Aph1a can cleave notch and amyloid precursor protein in the absence of nicastrin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:1648–1656. doi: 10.1523/JNEUROSCI.3826-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Mao G, Tan J, Dong Y, Cui M-Z, Kim S-H, Xu X. Identification of a New Presenilin-dependent z-Cleavage Site within the Transmembrane Domain of Amyloid Precursor Protein. J. Biol. Chem. 2004;279:50647–50650. doi: 10.1074/jbc.C400473200. [DOI] [PubMed] [Google Scholar]