Abstract
This study estimates the annual numbers of eight work-related cancers, total losses of quality-adjusted life years (QALYs), and lifetime healthcare expenditures that possibly could be saved by improving occupational health in Taiwan. Three databases were interlinked: the Taiwan Cancer Registry, the National Mortality Registry, and the National Health Insurance Research Database. Annual numbers of work-related cancers were estimated based on attributable fractions (AFs) abstracted from a literature review. The survival functions for eight cancers were estimated and extrapolated to lifetime using a semi-parametric method. A convenience sample of 8846 measurements of patients' quality of life with EQ-5D was collected for utility values and multiplied by survival functions to estimate quality-adjusted life expectancies (QALEs). The loss-of-QALE was obtained by subtracting the QALE of cancer from age- and sex-matched referents simulated from national vital statistics. The lifetime healthcare expenditures were estimated by multiplying the survival probability with mean monthly costs paid by the National Health Insurance for cancer diagnosis and treatment and summing this for the expected lifetime. A total of 3010 males and 726 females with eight work-related cancers were estimated in 2010. Among them, lung cancer ranked first in terms of QALY loss, with an annual total loss-of-QALE of 28,463 QALYs and total lifetime healthcare expenditures of US$36.6 million. Successful prevention of eight work-related cancers would not only avoid the occurrence of 3736 cases of cancer, but would also save more than US$70 million in healthcare costs and 46,750 QALYs for the Taiwan society in 2010.
Abbreviations: AF, Attributable fraction; CAREX, CARcinogen EXposure; DALY, Disability-adjusted life year; IARC, International Agency for Research on Cancer; LTHE, Lifetime healthcare expenditure; NHI, National Health Insurance; NHIRD, National Health Insurance Research Database; NMR, National Mortality Registry; QALE, Quality-adjusted life expectancy; QALY, Quality-adjusted life year; QOL, Quality of life; TCR, Taiwan Cancer Registry; WHO, World Health Organization
Keywords: Attributable fraction (AF), Lifetime healthcare expenditure (LTHE), Work-related cancer, Quality-adjusted life expectancy (QALE)
Highlights
-
•
A practical approach to estimate impact of work-related cancers is demonstrated.
-
•
3010 male and 726 female cancers were estimated work-related in Taiwan in 2010.
-
•
The impact included 46,750 QALYs annually, healthcare costs more than US$70 million.
1. Introduction
Cancer is one of the leading issues in global health due to its substantial reduction of life expectancy as well as quality of life (QOL). The financial burden associated with healthcare expenditures on cancer can have a massive impact on patients, their families, and the whole of society. Recently, the Global Burden of Disease Study has provided global and regional estimates of disability-adjusted life years (DALYs) lost due to many risk factors, including asbestos and other occupational exposures (Lozano et al., 2012). A study by the World Health Organization (WHO) estimated that approximately one fifth of all cancer cases could be attributed to occupations, and that these cases resulted in 1.3 million deaths each year worldwide (Prüss-Üstün et al., 2006). Over the past four decades, the International Agency for Research on Cancer (IARC) has also documented the workplace as a major source of exposure by having evaluated over 900 potential carcinogens (International Agency for Research on Cancer, World Health Organization). These estimates imply that a substantial proportion of incidences of cancer could possibly be prevented in work-related circumstances. However, practical recognition of cancer patients with occupational attribution for appropriate compensation remains a very challenging task (Langård and Lee, 2011) for several reasons. First, the long latency from exposure to diagnosis makes it difficult to identify a causal relationship (Baxter and Hunter, 2010). Second, it has been difficult to demonstrate the intensity and duration of exposure or dose of exposure to carcinogens, as there were generally few measurements at the beginning of industrialization because of a lack of knowledge and technology (World Health Organization, 1994). Third, the current theory of carcinogenesis is inclined to view cancer as a multi-step process. Moreover, genetic factors and personal behaviors, including cigarette smoking, may contribute synergistically to the occurrence of work-related cancer (Erren et al., 1999, European Commission, 2009).
In order to promote recognition of occupational cancer, estimates have been made of the proportion of cancers attributable to occupational exposure in both developed and developing countries (Parkin, 2011, Nurminen and Karjalainen, 2001, Mosavi-Jarrahi et al., 2009). To provoke policy consideration and improvements, however, we need to apply a common unit of measurement of outcomes and potential benefits of prevention, of which savings from expected years of life loss (EYLL) and lifetime healthcare expenditures (LTHE) have been proposed for the purpose of comparison of the impacts of work-related cancers (U.S. Environmental Protection Agency, 1997). However, it would be directly comparable with clinical healthcare technologies if the burden of work-related cancer could be measured with quality-adjusted life year (QALY) (Weinstein et al., 2009, Lipscomb et al., 2009, Kind et al., 2009), which could be estimated from the loss of quality-adjusted life expectancy (QALE) for different sites of cancer. In this study, we selected eight work-related cancers because of their high prevalence in Taiwan, which would be an initial step to estimate impacts from work-related cancers.
In Taiwan, there were a total of 463,703 overall cancer patients diagnosed in 2010; cancer related expenses accounted for 10.2% of the total National Health Insurance (NHI) healthcare expenditures that year (Bureau of Health Promotion Administration, Ministry of Heal, National Health Insurance Administration, Ministry of Healt). Unfortunately, the labor insurance system's compensation rate for occupational cancer is extremely low, with annual numbers ranging between 0 and 13 during 2003–2012 (Ministry of Labor in Taiwan). In this study, we aimed to estimate the annual numbers of work-related cancers, total losses of quality-adjusted life years (QALYs), and lifetime healthcare expenditures that potentially could be saved by improving occupational health in Taiwan.
2. Materials and methods
Before its commencement, this study was approved by the Institutional Review Board (IRB) of National Cheng Kung University Hospital (NCKUH) (B-ER-102-162). All patients with QOL data also provided signed informed consent. In brief, we first obtained the AFs of 8 work-related cancers based on Nurminen and Karjalainen's estimates (Nurminen and Karjalainen, 2001), which were followed by multiplying the above figures with the annual total numbers, loss-of-QALEs, and financial burdens for different cancers based on empirical data of Taiwan. Linkages of the Taiwan Cancer Registry (TCR), the National Mortality Registry (NMR), and the National Health Insurance Research Database (NHIRD) were performed to ascertain if a person is deceased, which were used to construct the lifetime survival functions of cancers for different organ-systems. As the NMR has been very comprehensive and all types of cancer are fully covered by the National Health Insurance with no copayment, the rates of loss to follow-up would be minimal or approaching 0. The QOL data were collected from patients who were treated in the Department of Oncology, NCKUH, and then were adjusted with survival function and summed up throughout lifetime to estimate QALE. We also adopted the method proposed by the World Health Organization (2004) to estimate the number of work-related lung cancer cases for validation.
2.1. Estimation of incidences of work-related cancers in Taiwan in 2010
The AFs of cancers caused by occupational exposure were taken directly from the comprehensive literature review conducted by Nurminen and Karjalainen, which summarized the most valid and/or suitable estimated relative risks for each cancer/substance combination (Nurminen and Karjalainen, 2001). The risk factors considered in this study for the 8 work-related cancers were based on literature review and listed as follows: polycyclic aromatic hydrocarbons, hydrocarbon solvents for cancer of oral cavity or cancer of esophagus, 8 lung carcinogens (arsenic, asbestos, beryllium, cadmium, chromium, diesel exhaust, nickel, and silica) for lung cancer, farming and rearing of livestock for stomach cancer, aflatoxins, chlorinated hydrocarbon solvents for liver cancer, asbestos, polycyclic aromatic hydrocarbons and other combustion products for colorectal cancer, ionizing radiation for breast cancer, and aromatic hydrocarbon solvents for cervical cancer.
Lung, colorectal, and liver cancers are leading causes of mortality in both genders, as well as breast and cervical cancers in females in Taiwan (Bureau of Health Promotion Administration, Ministry of Health and Welfare in Taiwan). Asbestos, a well-known occupational carcinogen, classified as IARC group I, is associated with increased risk of lung, oral, esophageal, stomach, and colorectal cancers (International Agency for Research on Cancer, World Health Or, Clin et al., 2009, Kjaerheim et al., 2005, Strand et al., 2010, Pukkala et al., 2009). Liver cancer is causally linked to occupational exposure to vinyl chloride (Ward et al., 2001) and hepatitis C (Yazdanpanah et al., 2005). Breast cancer has been recognized as a compensable occupational disease due to long-term shift work, as shown by epidemiological studies on nurses (Bonde et al., 2012). Cervical cancer may be associated with exposure to some hydrocarbon solvents (Weiderpass et al., 2001). We selected the eight cancers based on disease prevalence in Taiwan and the availability of data on quality of life.
We further obtained the numbers of incident cases of oral, esophageal, lung, stomach, colorectal, and liver cancers, as well as female cancers of breast and cervix, from the 2010 Annual Report of the Taiwan Cancer Registry (Bureau of Health Promotion Administration, Ministry of Health and Welfare in Taiwan), which were multiplied with the AFs to estimate the numbers of possible work-related cancers.
2.2. Estimation of total expected loss-of-QALE and healthcare expenditures
2.2.1. Study population and dataset
A total of 395,330 patients of the 8 work-related cancers who received care under the National Health Insurance (NHI) system during the period from 1998 to 2007, with pathologically verified cancers registered in the TCR were included and linked with the NMR until the end of 2010 to determine their vital status. They were then linked to the NHIRD to obtain reimbursement data. Then the identification number was encrypted before use for further analysis. The file contained detailed demographic data (including birthdate and gender), along with information regarding all payments and health care services provided for each patient. The data of diagnoses were coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The eight sites of cancer included oral cavity (ICD-9-CM code: 140-141), esophagus (ICD-9-CM code: 150), lung (ICD-9-CM code: 162), stomach (ICD-9-CM code: 151), liver (ICD-9-CM code: 155), colorectum (ICD-9-CM code: 153-154), and female cancers of breast (ICD-9-CM code: 174) and cervix (ICD-9-CM code: 180).
2.3. Estimation of life expectancy according to survival analysis and extrapolation
A semiparametric method was applied to extrapolate survival for up to 50 years to derive the lifetime survival function after the diagnosis of each cancer. Briefly, Kaplan–Meier's was first conducted for estimation of the survival function of cancer up to the end of follow-up (namely, 13 years); an age- and sex-matched reference population was generated using the Monte Carlo method from the life tables of the general population of Taiwan. The lifetime survival of the patients with cancer (up to 50 years) was obtained using linear extrapolation of a logit-transformed curve of the survival ratio between the cancer cohort and the reference population, under the assumption of a constant excess hazard (Lee et al., 2012, Fang et al., 2007, Hwang and Wang, 1999). Detailed methods and mathematical proofs were as described in our previous studies (Hwang and Wang, 1999, Hwang and Wang, 2004, Hwang et al., 1996).
2.4. Estimation of QALE and loss-of-QALE by measuring QOL via EQ-5D
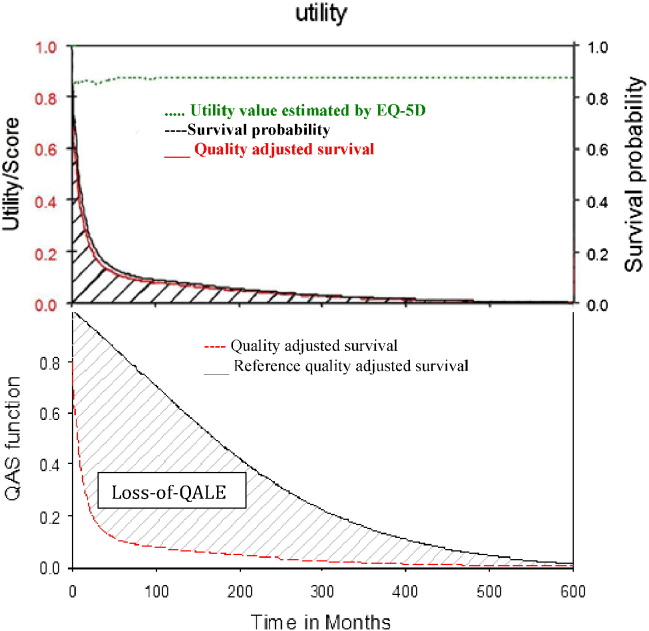
A convenient, cross-sectional sample with 8846 measurements was collected from the oncology clinic of National Cheng Kung University Hospital to estimate the utility value of QOL for these patients at different duration-to-dates from 2011 to 2013. Their QOL was assessed by administering the EQ-5D questionnaire through direct face-to-face interviews. This is a preference-based, generic instrument with five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), each with three levels of severity (no problems, some/moderate problems, and severe/extreme problems) (EuroQol group, 1990, Chang et al., 2007, Parkin et al., 2010). The measurement provides a utility value according to the population norm in Taiwan (Lee et al., 2013) that ranges from 0 to 1 based on the five-dimensional health-state classification, with 0 representing the worst health status and 1 representing perfect health. The duration-to-date is defined as the time interval between diagnosis and the time of EQ-5D assessment for each patient. In general, a cross-sectional, consecutive sample of patients was obtained, and a kernel-type smoothing method (using a moving average of the nearest 10% of the data) was performed to estimate the mean QOL throughout the duration-to-date (Hwang and Wang, 2004, Hwang et al., 1996). The QOL value after the end of the follow-up period was assumed to be the same as the average of the last 10% of measurements after smoothing. The lifetime survival probabilities over the course of a cancer under study were adjusted with the QOL utility values to obtain a quality-adjusted survival curve. The total area under this curve was the QALE (Hwang et al., 1996), as shown in the upper panel of Fig. 1. The loss-of-QALE for these patients was calculated by assuming a uniform utility of one for the age- and sex-matched reference subjects simulated from the hazard functions of life tables based on national vital statistics, and then subtracting the QALE of these patients, as shown in the lower panel of Fig. 1.
Fig. 1.
Estimation of quality-adjust life expectancies (QALE) as the area under the red curve (upper panel) and estimation of loss-of-QALE as the area between the curves (lower panel) for lung cancer (ICD-9-CM162).
2.5. Measurement of lifetime healthcare expenditures paid by the NHI for the eight types of cancer
NHIRD data provided every payment of the NHI for every incident cancer patient; we used this to estimate LTHE from the perspective of the single-payer system of NHI in Taiwan. The LTHE of a patient refers to all direct healthcare expenditures paid by the NHI from the date of cancer diagnosis until the date the patient is either deceased or censored. By retrieving the reimbursement data from the NHIRD, we were able to calculate the average healthcare expenditures spent by the patients with different types of cancer at time t, which can be added together to arrive at the lifetime figures after adjusting for the corresponding survival probability at time t. The effective sample size in each month in the follow-up period was applied for the calculation of the average monthly healthcare expenditure using SAS software. The LTHE per case was estimated by multiplying the monthly mean survival probability with the corresponding average monthly healthcare expenditure, adjusting for the annual 3% discount rate (Lee et al., 2012).
2.6. Validating incidences of work-related cancers by estimating the range of AF for occupational lung cancer based on the WHO method
The methodology involves five main steps: (1) estimating the proportion of the workforce exposed to eight identified lung carcinogens, stratified by industry sectors, (2) estimating numbers of workers ever exposed by multiplying the workforce exposed with different levels of intensity, (3) estimating the magnitude of relative risk of lung cancer by dividing it into high-exposure and low-exposure levels, (4) converting workers ever exposed to both high and low levels of carcinogens, and (5) calculating the AF using Levin's formula (Levin, 1953). They are briefly summarized as follows.
(1) The International Agency for Research on Cancer (IARC) has classified eight lung carcinogens: arsenic, asbestos, beryllium, cadmium, chromium, diesel exhaust, nickel, and silica. The proportions of workforces exposed to different carcinogens were estimated using the CARcinogen EXposure (CAREX) database (CAREX database. Helsinki (Finland), 1999); these proportions were directly applied in this study for the workforce employed in each sector. Then they were summed to arrive at the proportion of the total workforce exposed to lung carcinogens. (2) For lack of an official turnover rate of workers in Taiwan, we assumed a turnover rate of 4% for the base measure and a range of 3% to 10% for sensitivity analysis. And the figure for the workforce ever exposed was estimated by dividing into two groups: low intensity and high intensity. (3) A relative risk of 1.3 (95% CI: 1.4–1.8) was used for low-exposure level and 1.9 (95% CI: 1.7–2.1) for high-exposure level. Workers ever exposed to high versus low exposure levels were partitioned into 50%/50% groups for the base case, and into 10%/90% groups as the lower estimate for sensitivity analysis, respectively. (4) The rate of the economically active population was obtained from the website of Directorate-General of Budget, Accounting and Statistics, Executive Yuan, Taiwan (Directorate-General of Budget, 2015), which was 57.68% (69.4% for males and 46.02% for females) for quantification of number of workers ever exposed among the population ever exposed. (5) The AF of work-related cancer was calculated using Levin's equation (Levin, 1953) as follows:
where Pr(E) = proportion of the population exposed, and RR = relative risk of a specific exposed group.
3. Results
In total, we estimated 3010 male and 726 female cases of the eight cancers to be work related in Taiwan (Table 1). Among them, lung cancer accounted for the majority of the cancer cases (1942 males and 208 females), followed by 273 colon cancers and 271 liver cancers in males, and 173 liver cancers and 164 breast cancers in females. Colon cancer was the second-highest work-related cancer in males, but there was no incidence of work-related colon cancer in females due to a zero AF based on Nurminen and Karjalainen's estimate (Nurminen and Karjalainen, 2001).
Table 1.
Estimation of annual numbers of work-related cancers in Taiwan based on Taiwan Cancer Registry and assumption derived from literature review.
| Cancer site (ICD-9-CM code) | Percentage attributable to occupation |
Number of incident cancer in 2010 |
Estimated number of work-related cancer in 2010 |
|||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Oral cavity (140–141) | 1.0 | 0.3 | 3967 | 425 | 40 | 1 |
| Esophagus (150) | 6.4 | 0.2 | 2091 | 147 | 134 | 0 |
| Lung (162) | 29.0 | 5.3 | 6697 | 3918 | 1942 | 208 |
| Lung (162)⁎ | 4–18.9 | 2–10.5 | 6697 | 3918 | 268–1266 | 78–411 |
| Stomach (151) | 10.3 | 5.4 | 2415 | 1439 | 249 | 78 |
| Liver (155) | 3.5 | 5.3 | 7751 | 3272 | 271 | 173 |
| Colon (153) | 5.6 | 0.0 | 4871 | 3764 | 273 | 0 |
| Rectum (154) | 3.1 | 0.1 | 3272 | 2133 | 101 | 2 |
| Breast (174) | – | 1.7 | – | 9655 | – | 164 |
| Cervix (180) | – | 5.9 | – | 1680 | – | 99 |
| Total expected number | 31,064 | 26,433 | 3010a | 726a | ||
The estimation of percentage of cancers attributable to occupation is based on experts in WHO (2004) as a lower bound.
The total expected number of work-related cancers in 2010 were based on Nurminen and Karjalainen's (2001) estimates.
Table 2 shows the estimated QALE, loss of QALE, and LTHE for the eight types of cancer. Although cervical cancer patients were not the youngest at diagnosis, their life expectancy and QALE were the longest, i.e., 27.9 years and 25.9 QALY after diagnosis, respectively. Of the eight work-related cancers, lung cancer, liver cancer, and stomach cancer had the highest total loss-of-QALE of 28,463, 6973, and 3498 QALYs, respectively. Assuming a 3% discount rate, oral cancer and breast cancer represented the highest LTHE in males and females, or US$27,498 and US$28,180 per person, respectively. To sum up, prevention of these eight common cancers would save not only about 47,000 QALYs in the Taiwanese population but also about US$70 million in healthcare costs reimbursed by the NHI annually (Table 2).
Table 2.
Mean age at diagnosis, estimates of gender-stratified average quality-adjusted life expectancy (QALE), lifetime healthcare expenditures (LTHE), total loss-of-QALE, and total LTHE for patients with eight work-related cancers in Taiwan.
| Cancer site | Gender | Age (SD) |
LE (SE) |
QALE (SE) |
Average QALE loss | Total loss-of-QALE | Average LTHE (SE) | Total LTHE (∗ 103 USD) |
|---|---|---|---|---|---|---|---|---|
| Oral | Male | 51.9 (12.0) |
13.2 (0.0) |
11.7 (0.2) |
15.0 (0.2) |
600 | 27,498 (1979) |
1099.9 |
| Female | 59.5 (15.5) |
13.6 (0.1) |
13.3 (0.2) |
10.7 (0.2) |
10.7 | 24,147 (1590) |
24.1 | |
| Esophagus | Male | 59.7 (12.6) |
3.2 (0.0) |
2.8 (0.1) |
18.4 (0.1) |
2465.6 | 18,313 (625) |
2453.9 |
| Lung | Male | 68.2 (11.6) |
2.5 (0.0) |
2.2 (0.0) |
12.9 (0.0) |
25,051.8 | 16,607 (756) |
32,250.8 |
| Female | 64.9 (13.2) |
4.0 (0.0) |
3.4 (0.0) |
16.4 (0.1) |
3411.2 | 20,899 (713) |
4347.0 | |
| Lungb | Male | 68.2 (11.6) |
2.5 (0.0) |
2.2 (0.0) |
12.9 (0.0) |
3457–16,331 | 16,607 (756) |
4451–21,024 |
| Female | 64.9 (13.2) |
4.0 (0.0) |
3.4 (0.0) |
16.4 (0.1) |
1279–67,404 | 20,899 (713) |
1630–8589 | |
| Stomach | Male | 67.6 (13.2) |
6.4 (0.0) |
5.5 (0.3) |
10.1 (0.4) |
2514.9 | 17,435 (595) |
4341.3 |
| Female | 64.1 (15.2) |
9.0 (0.0) |
8.0 (0.4) |
12.6 (0.4) |
982.8 | 17,272 (589) |
1347.2 | |
| Liver | Male | 59.3 (13.3) |
5.8 (0.0) |
5.4 (0.1) |
15.9 (0.1) |
4308.9 | 19,759 (674) |
5354.7 |
| Female | 62.9 (12.9) |
6.1 (0.0) |
5.7 (0.1) |
15.4 (0.1) |
2664.2 | 19,725 (673) |
3412.4 | |
| Colorectum | Male | 65.4 (13.3) |
10.7 (0.0) |
9.9 (0.1) |
7.2 (0.1) |
2692.8 | 24,677 (1741) |
9229.2 |
| Female | 64.4 (14.1) |
12.9 (0.0) |
11.8 (0.1) |
8.5 (0.1) |
17 | 23,733 (1784) |
47.5 | |
| Breast | Female | 51.2 (12.1) |
23.6 (0.0) |
21.3 (0.2) |
9.6 (0.2) |
1574.4 | 28,180 (1452) |
4621.5 |
| Cervix | Female | 51.3 (14.6) |
27.9 (0.0) |
25.9 (0.3) |
4.6 (0.3) |
455.4 | 17,645 (1147) |
1746.9 |
| Total | 46,749.7a | 70,276.4a |
SD: standard deviation; SE: standard error of mean.
The total loss-of-QALE and TLHE were based on Nurminen and Karjalainen's (2001) estimates.
The estimation of percentage of cancers attributable to occupation is based on experts in WHO (2004) as a lower bound.
A total of 6.2 million males and 4.8 million females were employed in different economic sectors in Taiwan in 2010 (Table 3). Manufacturing represented the largest sector of the workforce, with a 28% share, followed by trade (23%) and service (23%). The proportions of the Taiwanese workforce exposed to the eight lung carcinogens under consideration were assumed to have similar exposure patterns to those estimated in CAREX. In total, 6.8% of the workforce was exposed to the carcinogens of interest. We further conducted a sensitivity analysis. In the workers with low previous exposure with turnover rate of 3, using the partitioning factors of 50%/50%, 10.1% were categorized as either high or low exposure levels; similarly, about 2% and 18% were categorized as high and low exposure levels, respectively, using partitioning factors of 10%/90%. The same calculation was applied to the workers with high previous exposure with turnover rate of 10. The ratio of the exposed workers to the population ever exposed was obtained by multiplying the rate of economically active population (= 57.8%); and the AF was estimated using the Levis' equation, which produced estimates of 4%–19%, as summarized in Table 4.
Table 3.
Proportion of Taiwanese workers in each economic sector stratified by gender.a
| Sectors (ISIC-2) | Proportion of workforce (%) | Proportion of male workforce (%) | Proportion of female workforce (%) |
|---|---|---|---|
| Agriculture | 7.79 | 5.62 | 2.17 |
| Mining | 0.11 | 0.09 | 0.02 |
| Manufacturing | 27.97 | 17.16 | 10.81 |
| Electrical | 0.38 | 0.32 | 0.06 |
| Construction | 8.76 | 7.84 | 0.92 |
| Trade | 22.79 | 11.81 | 10.98 |
| Transportation | 5.07 | 4.01 | 1.06 |
| Finance | 4.34 | 1.95 | 2.39 |
| Service | 22.78 | 10.92 | 11.86 |
| Total | 100.00 | 59.72 | 40.28 |
Based on Directorate General of budget accounting and labor statistics (Directorate-General of Budget, 2015).
Table 4.
Estimated attributable fractions (AFs) of occupational lung cancer based on the WHO approach with sensitivity analysis under different assumptions of workers ever exposed to different levels and intensity of carcinogens.
| % Workers ever exposed (turnover rate) | % Workers ever exposed with different levels (proportion factor) | % Population ever exposed by level | RRi | Pi × RRi | ||
|---|---|---|---|---|---|---|
| Unexposed | 88.3 | 1.0 | 0.883 | |||
| Low previous exposure | 20.1 (3) | 10.1 (50%) | 5.8 | 1.9 | 0.111 | |
| 10.1 (50%) | 5.8 | 1.3 | 0.076 | |||
| 2.0 (10%) | 1.2 | 1.9 | 0.022 | |||
| 18.1 (90%) | 10.5 | 1.3 | 0.136 | |||
| AF (%) | 4.0–6.5 | |||||
| Unexposed | 61.1 | 1.0 | 0.611 | |||
| High previous exposure | 67.0 (10) | 33.5 (50%) | 19.4 | 1.9 | 0.369 | |
| 33.5 (50%) | 19.4 | 1.3 | 0.253 | |||
| 6.7 (10%) | 3.9 | 1.9 | 0.074 | |||
| 60.3 (90%) | 35.0 | 1.3 | 0.455 | |||
| AF (%) | 12.3–18.9 | |||||
| Range of AF (%) | 4.0–18.9 |
RRi: relative risk for i stratum.
Pi: each population's exposure level.
AF = ΣPi RRi − 1 / ΣPi RRi based on WHO (2004).
4. Discussion
Although the total health impact of prevention from work-related cancer can be approximated by calculating the burden of disease with DALYs (Murray and Lopez, 1997, Murray et al., 2012, Ezzati et al., 2002), the results cannot be directly compared with the QALYs commonly used in healthcare services (Hwang and Wang, 2004, Salomon et al., 2012). Thus, we approached the question in an alternative way: How many QALYs would have been saved had the occurrence of a case of work-related cancer been successfully avoided? Namely, we estimated the loss-of-QALE and total LTHE due to work-related cancers from a societal perspective. Given limited resources, the total estimated QALYs and lifetime healthcare expenditures incurred by a specific type of cancer would be helpful in guiding the priority setting in resources allocation. Moreover, the potential impacts under the metrics of QALY and lifetime healthcare expenditures can be directly compared with outcomes of clinical diagnosis and treatment, and rehabilitation for cost-effectiveness assessment in health policy decision.
Our estimation is supported by following study strength. First, the survival data and reimbursement amounts were retrieved from direct inter-linkages of the following three databases: the TCR, the NMR, and the Registry of Catastrophic Illnesses from the NHIRD. Because every case registered as a catastrophic illness can be waived from co-payments, the cancer diagnoses are validated well in NHI (National Health Insurance Administration, Ministry of Health and Welfare in Taiwan, 2010). More than 90% of the cancer cases registered in the TCR provided histopathological evidence, except liver cancer (Bureau of Health Promotion Administration, Ministry of Health and Welfare in Taiwan). Thus, the diagnosis of cancer was generally accurate, and the linkage to the NMR assured the accuracy of the vital state. Second, since the cohorts were followed for 13 years, the estimation of life expectancies would be relatively accurate because patients with cancer other than cervix and breast usually do not survive for longer than 13 years according to the life expectancies (LE) listed in Table 2. Further, the semi-parametric method applied in this study has been mathematically proven to be correct if the assumption of constant excess hazard holds (Fang et al., 2007, Hwang and Wang, 1999). It was also found that the assumption holds for all eight cancers in our data analysis, which corroborates our previous 15-year follow-up study (Chu et al., 2008) and a recent Swedish study (Andersson et al., 2013). And third, since the NHI system in Taiwan is a single-payer system with universal coverage and because physicians are responsible for waiving the co-payment of cancer patients both in the clinic services and during hospitalization, the reimbursement data have been under careful monitoring to avoid waste or manipulation, and are generally accurate.
Because intensity of exposure, availability, and quality of worksite exposure vary from country to country, direct extrapolation of Nurminen and Karjalainen's estimates to Taiwan's situation might not be appropriate (Nurminen and Karjalainen, 2001). Thus, we conducted a sensitivity analysis based on WHO recommendations for different AF scenarios for male lung cancer, which resulted in a proportion between 4% and 19% (Table 4), which is lower than the 29% based on Nurminen and Karjalainen (Table 1). The discrepancy between the above two estimates may be partially explained by four reasons. First, Nurminen and Karjalainen attributed the synergistic effect of smoking with asbestos to occupational origins, which might elevate the AF up to as much as 14% (Nurminen and Karjalainen, 2001). Countries in the European Union tend to recognize work-relatedness for lung cancer as long as the asbestos exposure of an individual worker reaches the minimal requirement of intensity (European Commission, 2009). Second, in addition the use of the CAREX exposure matrix may be a lower bound exposure scenario for Taiwanese workers. For example, asbestos has been banned in most European countries for more than a decade (Parkin and Muir, 1992). Taiwan banned the use of asbestos in construction materials in 2008 and roof tiles in 2013, while that in newly manufactured brake-linings will be prohibited starting in 2018. Therefore, the proportion of the Taiwanese workforce exposed to asbestos in the construction and manufacturing sectors would be underestimated relative to that of the European Union. Third, since semiconductor manufacturing, which widely uses arsenic and silica, has been thriving in Taiwan for the last three decades, the proportions of the Taiwanese workforce exposed to arsenic and silica might be higher than those estimated by the CAREX database. Finally, because work-related cancer usually occurs at a younger age because of a potential synergistic interaction between occupational and non-occupational carcinogens (e.g., cigarette smoking), our loss-of-QALE might be an underestimation. For lack of data of exposure assessment of occupational carcinogens in industry sectors in Taiwan for the other cancer types, we did not perform sensitivity analysis for the 7 cancers.
4.1. Limitations
This study has following limitations. First, many new medicines and technology were not covered by Taiwan's NHI during the study period, such as some high-cost molecular targeted therapies. Therefore, the LTHE reimbursed by NHI may be underestimated for cancer treatment. We also did not include indirect medical costs, such as out-of-pocket money, which could account for more than 50% of the total costs of cancer treatment (American Cancer Society, 2012). The costs of productivity lost due to illness or premature death were not included in this study for lack of empirical data. Thus, further studies are needed to quantify these indirect medical costs and societal financial lost using human capital method or willingness to pay (Drummond et al., 2005). Second, some potential confounders as well as effect modifiers — such as income, educational level, and disease severity (i.e., cancer staging or associated co-morbidities) — were not available in these databases; we presumed these factors were distributed homogenously in patients with occupational and non-occupational cancers. A previous study showed both lower educational level and lower social economic status were related to early occupational exposure as well as shorter life expectancy (European Commission, 2009).
In conclusion, our study estimated the impact from potential numbers of work-related cancers in Taiwan. We have demonstrated a practical and feasible approach to quantify the total saving of QALYs and total LTHE for eight potentially preventable cancers that could be possibly eliminated by effective prevention programs.
Conflict of interest statements
The authors declare that there are no conflicts of interest.
Contributions
C-KL, M-C H, LJ-H L, and J-D W contributed to the conception and design of the work; C-KL and M-C H performed the data analysis. LJ-H L and C-KL drafted the manuscript and critically revised it with J-D W. LJ-H L and C-KL had equal contribution in the manuscript. All authors have contributed to the interpretation of the results and take public responsibility for its accuracy. Each author is confident in the validity of this work, has reviewed the final version of the manuscript, and approves it for submission.
Transparency document
Transparency document.
Acknowledgments
This work was supported by grants from the Institute of Labor, Occupational Safety and Health, Ministry of Labor, Taiwan (IOSH102-M3044), the Ministry of Health and Welfare, Taiwan (DOH 102-TD-C-111-003), the National Health Research Institutes (EO-102-PP04), as well as by funding from the Headquarters of University Advancement at National Cheng Kung University, which is sponsored by the Ministry of Education, Taiwan (D103-35A10). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Ministry of Health and Welfare or National Health Research Institutes.
Footnotes
The Transparency document associated with this article can be found, in the online version.
References
- American Cancer Society . American Cancer Society; Atlanta: 2012. Detailed Guide: Colon and Rectum Cancer. (c2014, < http://www.cancer.org/Cancer/ColonandRectumCancer/DetailedGuide/index>) [Google Scholar]
- Andersson T.M.L., Dickman P.W., Eloranta S., Lambe M., Lambert P.C. Estimating the loss in expectation of life due to cancer using flexible parametric survival models. Stat. Med. 2013;32:5286–5300. doi: 10.1002/sim.5943. [DOI] [PubMed] [Google Scholar]
- Baxter P., Hunter D. 10th ed. Hodder Arnold; London: 2010. Hunter's Diseases of Occupations. [Google Scholar]
- Bonde J.P., Hansen J., Kolstad H.A. Work at night and breast cancer — report on evidence-based options for preventive actions. Scand. J. Work Environ. Health. 2012;38:380–390. doi: 10.5271/sjweh.3282. [DOI] [PubMed] [Google Scholar]
- Bureau of Health Promotion Administration, Ministry of Health and Welfare in Taiwan 2010 Cancer Registry Report. http://www.hpa.gov.tw/BHPNet/Web/Stat/StatisticsShow.aspx?No=201305060001
- CAREX database. Helsinki (Finland) Finnish Institute of Occupational Health (FIOH) 1999. http://www.ttl.fi/en/chemical_safety/carex
- Chang T.J., Tarn Y.H., Hsieh C.L., Liou W.S., Shaw J.W., Chiou X.G. Taiwanese version of the EQ-5D: validation in a representative sample of the Taiwanese population. J. Formos. Med. Assoc. 2007;106:1023–1031. doi: 10.1016/S0929-6646(08)60078-9. [DOI] [PubMed] [Google Scholar]
- Chu P.C., Wang J.D., Hwang J.S., Chang Y.Y. Estimation of life expectancy and the expected years of life lost in patients with major cancers: extrapolation of survival curves under high-censored rates. Value Health. 2008;11:1102–1109. doi: 10.1111/j.1524-4733.2008.00350.x. [DOI] [PubMed] [Google Scholar]
- Clin B., Morlais F., Dubois B. Occupational asbestos exposure and digestive cancers—a cohort study. Aliment. Pharmacol. Ther. 2009;30:364–374. doi: 10.1111/j.1365-2036.2009.04050.x. [DOI] [PubMed] [Google Scholar]
- Directorate-General of Budget Accounting and Statics, Executive Yuan, Taiwan. 2015. http://win.dgbas.gov.tw/dgbas04/bc4/timeser/
- Drummond F., Sculpher M.J., Torrance G., O'Brien B.J., Stoddard G.L. third ed. Oxford University Press; 2005. Methods for the Economic Evaluation of Health Care Programmes. [Google Scholar]
- Erren T.C., Jacobsen M., Piekarski C. Synergy between asbestos and smoking on lung cancer risks. Epidemiology. 1999;10:405–411. doi: 10.1097/00001648-199907000-00008. [DOI] [PubMed] [Google Scholar]
- European Commission . European Communities; 2009. Information Notices on Occupational Diseases: A Guide to Diagnosis. Luxembourg. [Google Scholar]
- EuroQol group EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Ezzati M., Lopez A.D., Rodgers A., Vander Hoorn S., Murray C.J., Comparative Risk Assessment Collaborating Group Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- Fang C.T., Chang Y.Y., Hsu H.M. Life expectancy of patients with newly-diagnosed HIV infection in the era of highly active antiretroviral therapy. QJM. 2007;100:97–105. doi: 10.1093/qjmed/hcl141. [DOI] [PubMed] [Google Scholar]
- Hwang J.S., Wang J.D. Monte Carlo estimation of extrapolation of quality-adjusted survival for follow-up studies. Stat. Med. 1999;18:1627–1640. doi: 10.1002/(sici)1097-0258(19990715)18:13<1627::aid-sim159>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Hwang J.S., Wang J.D. Integrating health profile with survival for quality of life assessment. Qual. Life Res. 2004;13:1–10. doi: 10.1023/B:QURE.0000015299.45623.38. [DOI] [PubMed] [Google Scholar]
- Hwang J.S., Tsauo J.Y., Wang J.D. Estimation of expected quality adjusted survival by cross-sectional survey. Stat. Med. 1996;15:93–102. doi: 10.1002/(SICI)1097-0258(19960115)15:1<93::AID-SIM155>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, World Health Organization IARC Monographs on the Evaluation of Carcinogenic Risks of Humans. http://monographs.iarc.fr/ENG/Classification/latest_classif.php>
- Kind P., Lafata J.E., Matuszewski K., Raisch D. The use of QALYs in clinical and patient decision-making: issues and prospects. Value Health. 2009;12:S27–S30. doi: 10.1111/j.1524-4733.2009.00519.x. [DOI] [PubMed] [Google Scholar]
- Kjaerheim K., Ulvestad B., Martinsen J.I., Andersen A. Cancer of the gastrointestinal tract and exposure to asbestos in drinking water among lighthouse keepers (Norway) Cancer Causes Control. 2005;16:593–598. doi: 10.1007/s10552-004-7844-1. [DOI] [PubMed] [Google Scholar]
- Langård S., Lee L.J.H. Methods to recognize work-related cancer in workplaces, the general population, and by experts in the clinic, a Norwegian experience. J. Occup. Med. Toxicol. 2011;6(24) doi: 10.1186/1745-6673-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.J.H., Chang Y.Y., Liou S.H., Wang J.D. Estimation of benefit of prevention of occupational cancer for comparative risk assessment: methods and examples. Occup. Environ. Med. 2012;69:582–586. doi: 10.1136/oemed-2011-100462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.Y., Hung M.C., Hu F.C., Chang Y.Y., Hsieh C.L., Wang J.D. Estimating quality weights for EQ-5D (EuroQol-5 dimensions) health states with the time trade-off method in Taiwan. J. Formos. Med. Assoc. 2013;112:699–706. doi: 10.1016/j.jfma.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Levin M.L. The occurrence of lung cancer in man. Acta Unio Int. Contra Cancrum. 1953;9:531–541. [PubMed] [Google Scholar]
- Lipscomb J., Drummond M., Fryback D., Gold M., Revicki D. Retaining, and enhancing, the QALY. Value Health. 2009;12:S18–S26. doi: 10.1111/j.1524-4733.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- Lozano R., Naghavi M., Foreman K. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Labor in Taiwan Compensation number for occupational diseases. http://statdb.mol.gov.tw/statis/jspProxy.aspx?sys=210&kind=21&type=1&funid=q08061&rdm=ilZN5gji
- Mosavi-Jarrahi A., Mohagheghi M., Kalaghchi B., Mousavi-Jarrahi Y., Noori M.K. Estimating the incidence of lung cancer attributable to occupational exposure in Iran. Popul. Health Metrics. 2009;7:7. doi: 10.1186/1478-7954-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C.J., Lopez A.D. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- Murray C.J., Vos T., Lozano R. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- National Health Insurance Administration, Ministry of Health and Welfare in Taiwan National Health Insurance Statistics. 2010. http://www.nhi.gov.tw/webdata/webdata.aspx?menu=17&menu_id=1023&WD_ID=1043&webdata_id=4004
- Nurminen M., Karjalainen A. Epidemiologic estimate of the proportion of fatalities related to occupational factors in Finland. Scand. J. Work Environ. Health. 2001;27:161–213. doi: 10.5271/sjweh.605. [DOI] [PubMed] [Google Scholar]
- Parkin D.M., Muir C.S. Cancer incidence in five continents. Comparability and quality of data. IARC Sci. Publ. 1992;120:45–173. [PubMed] [Google Scholar]
- Parkin D.M. 14 Cancers attributable to occupational exposures in the UK in 2010. Br. J. Cancer. 2011;105:S70–S72. doi: 10.1038/bjc.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin D., Rice N., Devlin N. Statistical analysis of EQ-5D profiles: does the use of value sets bias inference? Med. Decis. Mak. 2010;30:556–565. doi: 10.1177/0272989X09357473. [DOI] [PubMed] [Google Scholar]
- Prüss-Üstün A., Corvalán C., World Health Organization . World Health Organization; Geneva, Switzerland: 2006. Preventing Disease Through Healthy Environments: Towards an Estimate of the Environmental Burden of Disease. [Google Scholar]
- Pukkala E., Martinsen J., Lynge E. Occupation and cancer- follow-up of 15 million people in five Nordic countries. Acta Oncol. 2009;48:646–790. doi: 10.1080/02841860902913546. [DOI] [PubMed] [Google Scholar]
- Salomon J.A., Vos T., Hogan D.R. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L.A., Martinsen J.I., Koefoed V.F., Sommerfelt-Pettersen J., Grimsrud T.K. Asbestos-related cancers among 28,300 military servicemen in the Royal Norwegian Navy. Am. J. Ind. Med. 2010;53:64–71. doi: 10.1002/ajim.20778. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Office of Pollution Prevention and Toxics, Economics Exposure and Technology Division, Regulatory Impacts Branch; Washington, DC: 1997. Cost of Illness Handbook. (< http://yosemite.epa.gov/ee/epa/eed.nsf/pages/InternetLinksSubject.html?OpenDocument&Count=300&ExpandView>) [Google Scholar]
- Ward E., Boffetta P., Andersen A. Update of the follow-up of mortality and cancer incidence among European workers employed in the vinyl chloride industry. Epidemiology. 2001;12:710–718. doi: 10.1097/00001648-200111000-00021. [DOI] [PubMed] [Google Scholar]
- Weiderpass E., Pukkala E., Vasama-Neuvonen K. Occupational exposures and cancers of the endometrium and cervix uteri in Finland. Am. J. Ind. Med. 2001;39:572–580. doi: 10.1002/ajim.1056. [DOI] [PubMed] [Google Scholar]
- Weinstein M.C., Torrance G., McGuire A. QALYs: the basics. Value Health. 2009;12:S5–S9. doi: 10.1111/j.1524-4733.2009.00515.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization Occupational health. Global strategy on occupational health for all: the way to health at work. Recommendation of the second meeting of the WHO Collaborating Centres in Occupational Health Organization in Beijing, China. 1994. http://www.who.int/occupational_health/globstrategy/en/
- World Health Organization . Occupational Carcinogens, Environmental Burden of Disease Series, No. 6. 2004. Protection of the human environment Geneva.http://www.who.int/quantifying_ehimpacts/publications/en/ebd6.pdf [Google Scholar]
- Yazdanpanah Y., De Carli G., Migueres B. Risk factors for hepatitis C virus transmission to health care workers after occupational exposure: a European case–control study. Clin. Infect. Dis. 2005;41:1423–1430. doi: 10.1086/497131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.